Albumin (Alb), one of the most abundant proteins in the body, activates a proton channel (hHv1), which allows sperm to penetrate and fertilize an egg and white blood cells to secrete large amounts of inflammatory mediators to fight infection, according to a new study led by the University of California, Irvine.
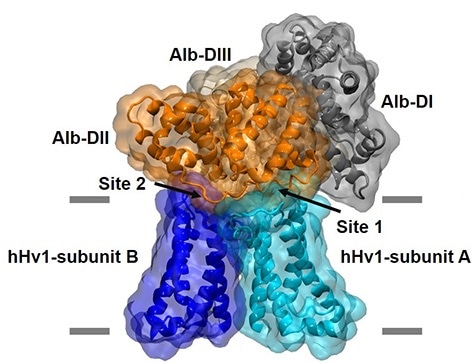
The model of the Alb-hHv1 complex was generated by molecular dynamics simulations (MDS) using a crystal structure of Alb and the reported modeling structure of the hHv1 channel. A single Alb molecule has three domains and two sites in domain II bind to the two identical subunits that form the channel. The final model was equilibrated and refined by MDS in a hydrated lipid bilayer using residue-residue interactions suggested by in silico docking and validated by studies of Alb and hHv1 point mutants using electrophysiology and fluorescence resonance energy transfer spectroscopy. Image Credit: University of California, Irvine
The study was published in Nature Communications and is titled “Direct activation of the proton channel by albumin leads to human sperm capacitation and sustained release of inflammatory mediators by neutrophils.”
The physiological relationship between Alb and human voltage-gated proton channels (hHv1), which are both important in cell biology in health and sickness, was investigated. They also established the mechanism through which Alb activates hHv1 by binding directly to it.
This study explains how sperm fertilize and neutrophils release mediators in the innate immune response, providing a new role for Alb in physiology that will be active in the various tissues that express hHv1.
We found that the interaction of Alb and hHv1 activates sperm when they leave semen and enter the female reproductive tract because Alb is low in semen and high in the reproductive tract. We now understand why albumin supplementation improves IVF. We also found the same Alb/hHv1 interaction allows the white blood cells called neutrophils to produce and secrete the inflammatory mediators that kill bacteria and fight infection. However, it’s important to note that the inflammatory response itself can lead to disease.”
Ruiming Zhao, PhD, Study First Author, Department of Physiology & Biophysics, UCI School of Medicine
Alb’s crucial stimulatory involvement in the physiology of sperm and neutrophils via hHv1 predicts that Alb will have as-yet-unidentified enhancing or detrimental implications in other tissues, such as the central nervous system, heart, and lungs, and will influence breast and gastrointestinal malignancies.
It is exciting to discover that a common protein has the power to activate the proton channel. This finding suggests new strategies to block or enhance fertility, and to augment or suppress the innate immune response and inflammation.”
Steve A. N. Goldstein, MD, PhD, Study Senior Author and Vice Chancellor, Health Affairs, UCI
Goldstein is also a distinguished professor in the School of Medicine Departments of Pediatrics and Physiology & Biophysics, and the new School of Pharmacy and Pharmaceutical Sciences.
Apart from the capacitation of sperm and the innate immune responses studied, hHv1 is also involved in a wide range of biological activities. The channels have important roles in cancer cell proliferation, tissue damage after ischemic stroke, and kidney hypertensive injury.
Alb’s potentiation of hHv1 revealed in the research will be extensive, tissue-dependent, and play both beneficial and harmful roles in human physiology because Alb is ubiquitous at levels that fluctuate in different human compartments in health and sickness.
We have modeled the structural basis for binding of Alb to the channel that leads to activation and changes in cellular function, and we are now conducting in vivo studies of viral and bacterial infections. Our next steps include studies of the effects of inhibitors of the Alb-hHv1 interaction on infection, inflammation and fertility.”
Steve A. N. Goldstein, MD, PhD, Study Senior Author and Vice Chancellor, Health Affairs, UCI
Source:
Journal reference:
Zhao, R., et al. (2021) Direct activation of the proton channel by albumin leads to human sperm capacitation and sustained release of inflammatory mediators by neutrophils. Nature Communications. doi.org/10.1038/s41467-021-24145-1.