Stomata, the small microscopic pores present on leaves in plants, aids in regulating the influx of carbon dioxide for photosynthesis. Plants also utilize stomata to prevent water loss and withering away during drought.
Transpiration. Image Credit: Kazakova Maryia/Shutterstock.com
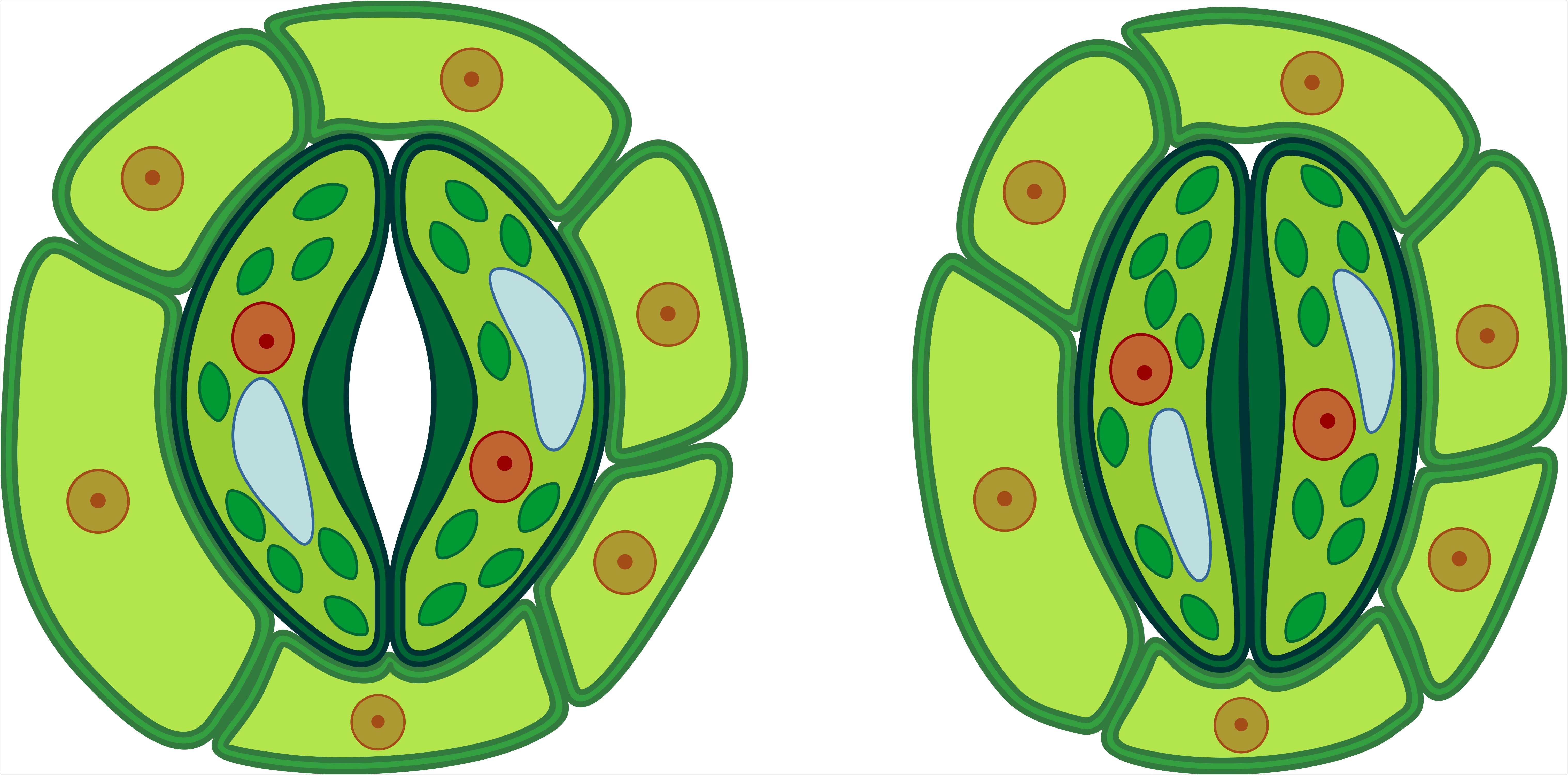
Two guard cells surround the stomatal pores. When the internal pressure of these cells decreases, they loosen, resulting in closed pores. Alternatively, when the pressure is high, the cells move apart, resulting in widened pores.
The stomatal movements are controlled by the guard cells, the signaling pathways of which are much complex that it is hard for humans to work with them directly. Yet, scientists from the Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany, came up with a technique to regulate stomatal movements remotely—by employing light pulses.
Light-sensitive protein from algae used
The scientists adopted a technique from optogenetics—they introduced a light-sensitive switch into the guard cells of tobacco plants. The technique has been employed efficiently in animal cells; however, it is still in infancy in plant cells.
The researchers headed by JMU biophysicist and guard cell expert Professor Rainer Hedrich outlined their research findings in the journal Science Advances. Other JMU researchers involved in the study included Shouguang Huang, the first author of the study, and Kai Konrad and Rob Roelfsema.
The researchers employed a light-sensitive protein from the alga Guillardia theta as a light switch, called the anion channel ACR1 from the group of channelrhodopsins. Depending on the light pulses, the switch ascertains that chloride flows out of the guard cells followed by potassium. The guard cells lose their internal pressure, relax, and close the pore in around 15 minutes.
“The light pulse is like a remote control for the movement of the stomata,” states Hedrich.
Anion channel hypothesis confirmed
By exposing ACR1 to light, we have bridged the cell’s own signaling chain, thus proving the hypothesis that the opening of anion channels is essential and sufficient for stomatal closure.”
Rainer Hedrich, Professor, Guard Cell Expert, and Biophysicst, Würzburg University
Light exposure almost fully prohibited transpiration in plants.
This awareness helps cultivate plants with higher anion channels in the guard cells. Plants primed in this manner would close their stomata as a quicker response to the imminent heat waves and could cope better during drought.
Plant anion channels are activated during stress; this process is dependent on calcium. In a follow-up optogenetics project, we want to use calcium-conducting channelrhodopsins to specifically allow calcium to flow into the guard cells cell through exposure to light and to understand the mechanism of anion channel activation in detail.”
Rainer Hedrich, Professor, Guard Cell Expert, and Biophysicst, Würzburg University
The findings from Würzburg also prove beneficial for basic scientific research. “Our new optogenetic tool has enormous potential for research. With it, we can gain new insights into how plants regulate their water consumption and how carbon dioxide fixation and stomatal movements are coupled,” ascertains Hedrich.
Source:
Journal reference:
Huang, S., et al., (2021) Optogenetic control of the guard cell membrane potential and stomatal movement by the light-gated anion channel GtACR1. Science Advances. doi.org/10.1126/sciadv.abg4619.