A new study has unraveled a causal gene (Necdin, NDN) in autism model mice that exhibit the chromosomal abnormality known as copy number variation.
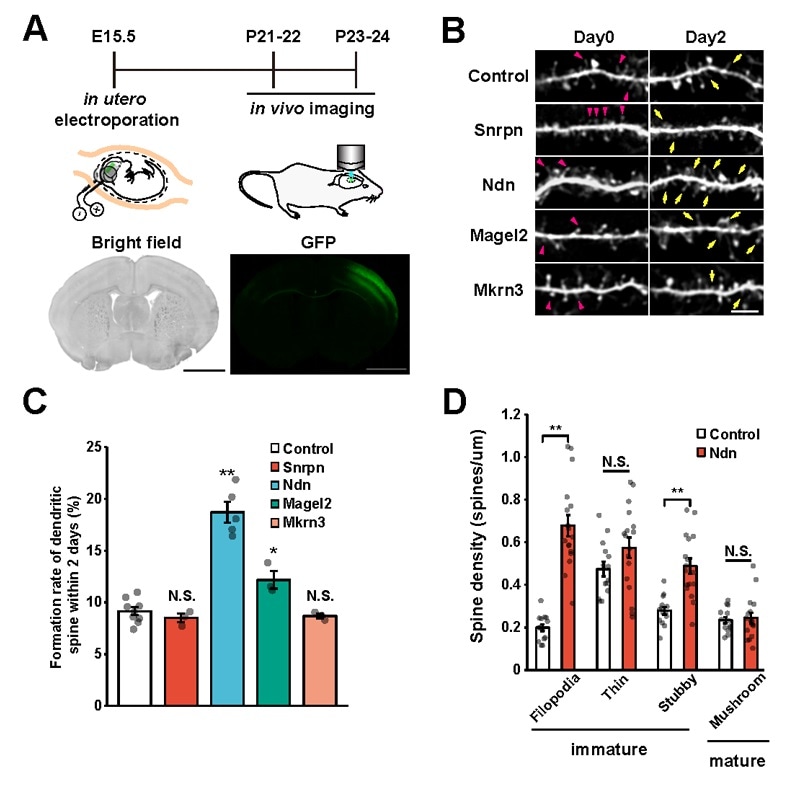
A: Illustration of the experiment. Green fluorescent proteins were introduced so that the target gene and the entire neuron could be observed. B: Each target gene was separately introduced into the cerebral cortex and dendritic spine dynamics were tracked for a period of 2 days (on the 21st and 22nd days after birth). Yellow arrows indicate newly formed spines and red arrowheads indicate spine elimination. C: The quantified results of B in graph form. D: Spine morphology classification data on the spines that formed when Ndn was introduced. Filopedia, a type of immature spine formation, significantly increased upon Ndn introduction. Image Credit: Kobe University.
The findings were achieved by a group of researchers including Professor Toru Takumi (who is also a Senior Visiting Scientist at RIKEN Center for Biosystems Dynamics Research) and Assistant Professor Kota Tamada from Kobe University, both from the Physiology Division in the Graduate School of Medicine.
The aim of the researchers is to throw more light on the molecular mechanism of the NDN gene to contribute toward developing novel treatment approaches for developmental disorders such as autism.
These findings of the study were published in Nature Communications on July 1st, 2021.
Research background
There has been a considerable increase in the number of patients diagnosed with autism (autism spectrum disorder). However, various aspects of this developmental disorder are still obscure. Its causes can be divided into environmental and genetic factors.
In the genetic factors, researchers have found specific copy number variations in autistic patients; for instance, chromosome 15q11-q13 duplication. Such abnormalities in the 15q11-q13 region are classified into paternally derived and maternally derived chromosomal duplication cases.
It has been considered that the Ube3a gene induces maternally derived chromosomal duplication. But it is not clear which gene is crucial for paternally derived duplication.
The team was previously successful in creating a mouse model of 15q11-q13 duplication (15q dup mouse). They used this mouse model to identify various abnormalities in paternally derived chromosomal duplication cases, such as autism-like behaviors and abnormalities in dendritic spine formation.
But the researchers could not identify the gene responsible for autism-like behavior. The reason is this region includes several non-coding RNA molecules and genes that code proteins.
Research methodology
In the case of 15q dup mice, the number of genes is vast as the duplication extends to the 6Mb region. Earlier studies revealed that behavioral abnormalities were not driven by maternally derived chromosomal duplication, and thus around 2Mb was excluded.
In the case of the remaining 4Mb, the team initially created a new 1.5Mb duplication mouse model and analyzed behavior abnormalities. Using the results, they could identify any autism-like behavioral abnormalities in 1.5Mb duplication mice. Thus, they excluded this 1.5Mb and were left with three protein-encoding genes as potential candidates.
Then, the researchers introduced these three genes individually into the cerebral cortex of mice through in utero electroporation. They used a two-photon microscope to measure the spine turnover rate (formation and removal of dendritic spines over a two-day period) in vivo. They found that there was a substantial increase in the number of spines when the Ndn gene was introduced.
Moreover, when these spines were morphologically classified, it was found that most were immature. This shows that the Ndn gene controls the formation and maturation of dendritic spines at the time of the developmental stage.
The researchers used CRISPR-Cas9 to subsequently eliminate one copy of the Ndn gene from the 15q dup mouse model to produce mice with a normalized genomic copy number for this gene (15q dupΔNdn mouse). This model was used to show that the abnormalities found in 15q dup mice (decreased inhibitory synaptic input and abnormal spine turnover rate) could be ameliorated.
Finally, the team examined whether the autism-like behaviors observed earlier in 15q dup mice (such as increased perseveration, increased anxiety in a new environment, and reduced sociability) were seen in 15q dupΔNdn mice. They demonstrated that in a majority of the behavioral test results for 15q dupΔNdn mice, abnormal behaviors associated with perseveration and sociability were ameliorated.
Further research
This study showed that the NDN gene in 15q dup autism model mice not only plays a crucial role in autism-like behaviors but also has an impact on aspects like excitation/inhibition imbalance in synaptic dynamics and the cerebral cortex. As a next step, the researchers look forward to clarifying the functions of the NDN gene.
Through artificial control of these functions or by determining and regulating their downstream factors, the team hopes to gain insights into the onset mechanism of developmental disorders, such as autism, and develop new treatment approaches.
Source:
Journal reference:
Tamada, K., et al. (2021) Genetic dissection identifies Necdin as a driver gene in a mouse model of paternal 15q duplications. Nature Communications. doi.org/10.1038/s41467-021-24359-3.