In the process of drug development, solving structures of potential therapeutics with X-ray diffraction (XRD) has been a presumed, critical step so far.
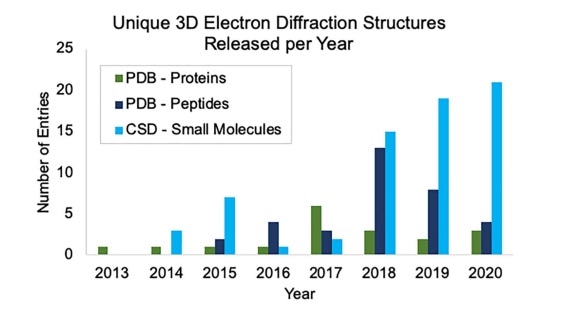
The growth of unique structure depositions by year in the Protein Data Bank (PDB) and Cambridge Structural Database (CSD) determined by MicroED or related 3D electron diffraction techniques. Depositions into the PDB are broken up into proteins and peptides. Proteins were defined as having more than 50 amino acids. Image Credit: Bruhn, J. et al. Front. Mol. Biosci., 2021 8, 354.
However, a new study by a research team headed by NanoImaging Services demonstrates how microcrystal electron diffraction (MicroED) has been growing to achieve the structures of potential pharmaceuticals.
Three-dimensional crystal structures that display the relative positions of bonds, atoms, and intramolecular interactions are crucial to understanding the reactivity, stability, solubility, and, ultimately, suitability for pharmaceutical use. In general, pharmaceutical researchers use X-ray diffraction (XRD)—where single-crystal XRD is preferred—to solve crystal structures.
However, XRD often necessitates large (100 µm or larger), well-ordered crystals and thousands of familiar active pharmaceutical ingredients (APIs) occur only as crystalline powders that do not readily form large crystals.
Growing large crystals is a huge bottleneck for those interested in determining crystal structures. MicroED can work with crystals of almost any size as it is generally fairly straightforward to break large crystals into a size suitable for MicroED.”
Dr Jessica Bruhn, Study Author and Scientific Group Leader – MicroED, NanoImaging Services
Advancements in the automated collection and processing of data have paved the way to higher interest in electron diffraction as an alternative to XRD. Electron diffraction is similar to XRD but that it involves using a beam of electrons in the place of X-rays to obtain structures.
Electrons tend to interact with matter readily, and thus MicroED can help solve high-resolution crystal structures from sub-micron-sized crystals. It is mainly intriguing for small-molecule drugs, several of which tend to readily form microcrystals. Moreover, the technology supports the drug discovery phase when there are very limited sample quantities.
During the development phase, scientists can employ it to identify structures of reaction products and by-products, which can help manage synthesis methods and make informed production decisions.
Single-crystal X-ray diffraction is faster, cheaper and easier to access compared to electron diffraction today. However, I do expect to see electron diffraction determining more and more structures inaccessible to X-rays, such as those of transient polymorphs, helping to expand the breadth of crystal structures that can be determined.”
Dr Jessica Bruhn, Study Author and Scientific Group Leader – MicroED, NanoImaging Services
Polymorphs are defined as crystalline structures that exhibit the same chemical composition but a distinct molecular arrangement with different lattice properties, for example, diamond and graphite. A majority of the active pharmaceutical ingredients (APIs) are considered to occur in more than one polymorphic form, which can lead to dramatically different drug properties.
Successful formulation of drugs necessitates the selection of the optimal polymorph, which in turn necessitates structures that can be easily solved. Besides, a majority of the drug substances developed currently have poor solubility.
By identifying the structures of several different forms that can be adopted by an API (such as hydrates, polymorphs, solvates, co-crystals, etc.), researchers can better design optimal crystal forms with improved pharmacokinetic properties.
To create their MicroED pipeline, the team investigated the prevalence of available MicroED data, such as data stored in the Cambridge Structural Database (CSD). The CSD hosts small-molecule organic and metal-organic experimental crystal structures, where the entries are enriched and annotated by experts.
Nearly 98% of the structures in the CSD are obtained from laboratory X-ray diffractometers. However, the CSD hosts an increasing number of 3D electron diffraction datasets, such as those solved using MicroED. At present, there are more than 100 exclusive datasets identified using electron diffraction in the CSD’s June 2021 web and desktop offerings.
In the last three years, there has been a quick increase in the number of electron structures in the CSD, and the Cambridge Crystallographic Data Centre (CCDC) is devoted to supporting researchers across the globe who have been depositing and sharing their MicroED data internationally.
Electron diffraction is truly one of the most exciting and rapidly evolving areas of structural science. Recent publications already show how it could help to speed up the development of new drugs, and we are eagerly anticipating how it might impact the volume and breadth of data we are able to share through the CSD.”
Suzanna Ward, Head of Database, CCDC
“I think we have an interesting journey ahead of us, and it will be intriguing to see how 3D electron diffraction will be utilized in both industry and academia in the coming years,” concluded Ward.
Source:
Journal reference:
Bruhn, J. F., et al. (2021) Small Molecule Microcrystal Electron Diffraction for the Pharmaceutical Industry–Lessons Learned From Examining Over Fifty Samples. Frontiers in Molecular Biosciences. doi.org/10.3389/fmolb.2021.648603.