During the invasion of pathogens into the human body, a prompt response is needed. There are special immune cells at the forefront of the immune response, and they reside in different tissues like the liver, lungs, intestines, and skin—where they combat invaders at the initial stage. They are known as innate lymphoid cells or ILCs.
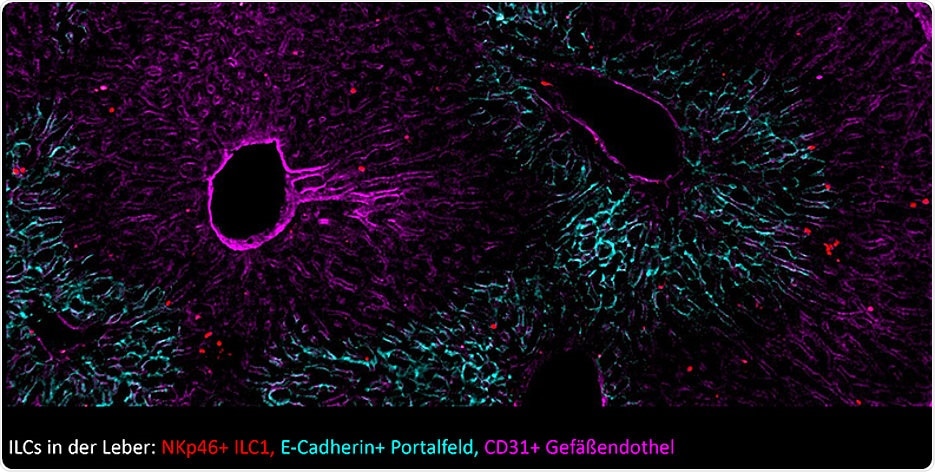
Liver tissue with innate lymphoid cells (ILCs), visualized here as red dots. Image Credit: Ye Ouyang / University of Würzburg.
These cells exhibit a special property—they do not have to be signaled first in lymphoid organs, like the majority of the immune cells, to migrate to their place of action. Alternatively, they settle in the organs and tissues immediately after birth and remain there forever.
Single-cell mRNA atlas of ILC1
ILCs can occur in tissues from immature precursor cells and mature into functional immune cells. This has now been demonstrated by researchers of the Max Planck Research Group at the Institute of Systems Immunology of Julius-Maximilians-Universität (JMU) Würzburg in Bavaria, Germany. Until recently, the maturation proceeds were unclear.
We wanted to understand how immature ILCs become effector cells that can, for example, kill tumor cells or fight infections with the help of cytokines.”
Georg Gasteiger, Professor, Chair, and Head, Max Planck Research Group, Institute of Systems Immunology, Julius-Maximilians-Universität
To achieve this, the Würzburg scientists examined the group of ILC1s that undertake a role in viral infections and tumor defense. The researchers recorded all mRNA molecules of individual ILC1s in the liver and developed a virtual cell atlas from these examinations.
Division of tasks: Supply, helper, and killer cells
On the basis of these “molecular fingerprints,” the scientists identified that there are specialized cells inside the ILC1 that share their tasks: “We found cells that can multiply very quickly and thus provide for replenishment of ILCs. In the process, they specialize into so-called helper or killer ILCs.”
Gasteiger and co-workers identified that the helper cells produce a broad range of messenger substances (cytokines) that play a role in the initial phase of infections, for instance. However, the killer cells are equipped with molecules that enable them to identify and kill tumor cells.
“Until now, it was thought that these cells were different types of ILCs,” details Christin Friedrich, a postdoctoral researcher from Gasteiger’s team. He is the first author of the study that was published in the Nature Immunology journal.
But our data show that they are different degrees of specialization that can arise in each organ from the same supply troops.”
Christin Friedrich, Study First Author and Postdoctoral Researcher, Max Planck Research Group, Institute of Systems Immunology, Julius-Maximilians-Universität
Can killer ILCs be made therapeutically useful?
“Interestingly, however, ILCs only develop into killer cells in some tissues, although our data show that they have the potential to do so in all tissues,” elaborates Gasteiger.
We have initial evidence that this development is actively suppressed in some tissues, possibly to avoid tissue damage or inflammation. We now want to understand how we can therapeutically activate the killer cells, for example, to improve immune control of developing tumors and metastases. We also want to investigate which molecules the ILCs can use to recognize tumors and how they behave in different tissues during infections.”
Georg Gasteiger, Professor, Chair, and Head, Max Planck Research Group, Institute of Systems Immunology, Julius-Maximilians-Universität
Transcription factor Hobit drives specialization
“Our work shows how the transcription factor Hobit drives specialization to mature effector cells. It is exciting that Hobit is also expressed in other killer cells of the human immune system. Based on our results, the function of Hobit in these defense cells can now be explored, how they mature and how they might be induced to fight tumors in different tissues,” concludes Christin Friedrich.
Source:
Journal reference:
Friedrich, C., et al. (2021) Effector differentiation downstream of lineage commitment in ILC1s is driven by Hobit across tissues. Nature Immunology. doi.org/10.1038/s41590-021-01013-0.