Proteins are the messengers, workers, managers, and directors of mostly all intra- and inter-cellular functions in the human body. Hence all advances in pharmaceuticals, biology, and associated areas pivot on attaining a basic realization of the mechanism of proteins.
New theoretical calculations reveal that it is not the repulsion force between the hydrophobic part of proteins and water that drives the protein into forming a folded structure. Rather, the special attraction between the hydrophobic molecules, called van der Waals force, is what causes the folding. Image Credit: Dr Tomonari Sumi (Okayama University) and Dr Hiroshi Imamura (Ritsumeikan University).
For many decades, a major theory that has informed scientific and technological advances in biosciences is the conventional theory on the process underlying protein folding. But two researchers from Okayama University and Ritsumeikan University in Japan recently disproved it. The observations have been published in the Protein Science journal.
Proteins initially develop as a chain of compounds named amino acids in human cells. Some portions of this chain are easily water-soluble—“hydrophilic.” While other sections were thought to be water-repelling—“hydrophobic”—until recently. The Japanese scientists questioned this hydrophobicity.
Interactions among the amino acids and between amino acids and water result in the successful folding of the protein on itself.
The folding patterns are pre-programmed for every protein, and the folded structures are designed to allow certain interactions with other molecules for particular functions. Alternately stated, the proteins shift from an unfolded state to a folded one to operate appropriately. The misfolding of proteins can result in numerous diseases like cystic fibrosis, Alzheimer’s, or even allergies.
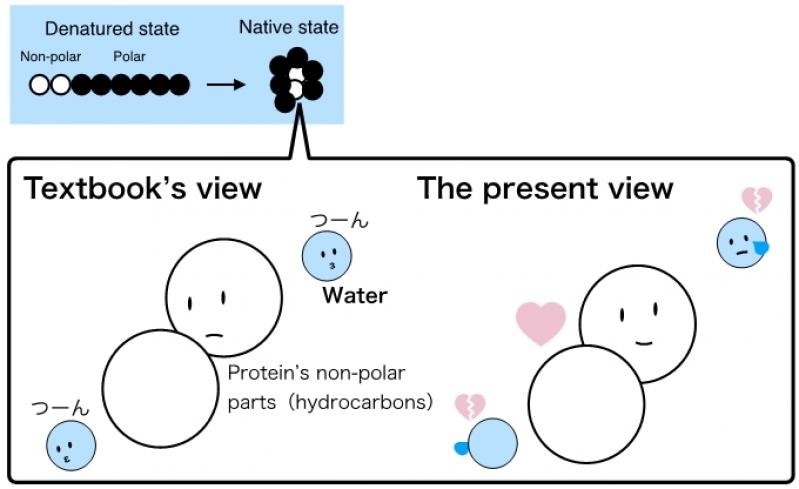
In 1959, a theory was put forth on the processes behind protein folding. This conventional theory postulates that hating water, i.e., the hydrophobic parts when exposed to water, is energetically disadvantageous and hence the proteins fold in such a way that they hide the hydrophobic parts into the interior of the protein structure. This indicates that the hydrophobic repulsive force stabilizes the folded protein structure.
For many years, numerous findings demonstrated that this theory was too broad and simplistic, and hence, it was polished to encompass consideration of the force of attraction between the hydrophobic parts (the van der Waals force). Yet, the hydrophobic repulsion remained to be regarded as the force underlying the folding.
As stated by the two Japanese researchers, Dr. Tomonari Sumi (Okayama University) and Dr. Hiroshi Imamura (Ritsumeikan University), this theory overlooked another major contribution in play—the cavity formation energy (or energy required to form a space to house the buried parts into the interior of protein).
To unravel the input of all the interactions on protein folding stability, the researchers carried out theoretical calculations employing a novel computational technique named the reference-modified density functional theory.
The researchers employed a typical model for calculating hydrophobic interactions—the coiled-coil protein GCN4-p1—which folds into a two-layered structure named the leucine zipper (as it appears like a closed zipper). The researchers examined the energies needed for the folding and unfolding mechanisms.
The observations were a surprise.
Our study shows that when the proteins are unfolded, the interactions of the hydrophobic parts with water (the van der Waals force) actually stabilizes the unfolded structure. So, the classical view of the hydrophobic groups ‘hating’ water and thus causing the protein to fold is not appropriate."
Dr Tomonari Sumi, Okayama University
“Instead, the fact is that the van der Waals force between the hydrophobic parts is stronger than the ‘effective’ water-mediated repulsive force between them, and so, the proteins fold over. It is the direct attraction between the molecules rather than the repulsion with water that causes the folding. The intramolecular van der Waals force is the stabilizing force in the folded structure,” adds Dr Sumi.
The researchers expect that these observations would provoke further analyses into this phenomenon and an exploration into the science the conventional theory has provided. They also anticipate that this seeps down to core education and textbooks and alter the interpretations of the phenomenon.
In our results, the ‘visible’ phenomenon, which is that the proteins fold to stably tuck the hydrophobic groups in the core of the structure, remains the same, but our understanding of the underlying mechanism must change."
Dr Hiroshi Imamura, Ritsumeikan University
The researchers anticipate that their findings would be accepted extensively and the ripple effects of that are observed in pharmaceutical and engineering applications too.
Source:
Journal reference:
Sumi, T & Imamura, H (2021) Water-mediated interactions destabilize proteins. Protein Science. doi.org/10.1002/pro.4168.