Recent research states that the number of secretory granules (SGs) in tyrosine hydroxylase-positive neurons and the marker proteins secretogranin III substantially lowered in the substantia nigra and striatum regions of mice exposed to 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine.
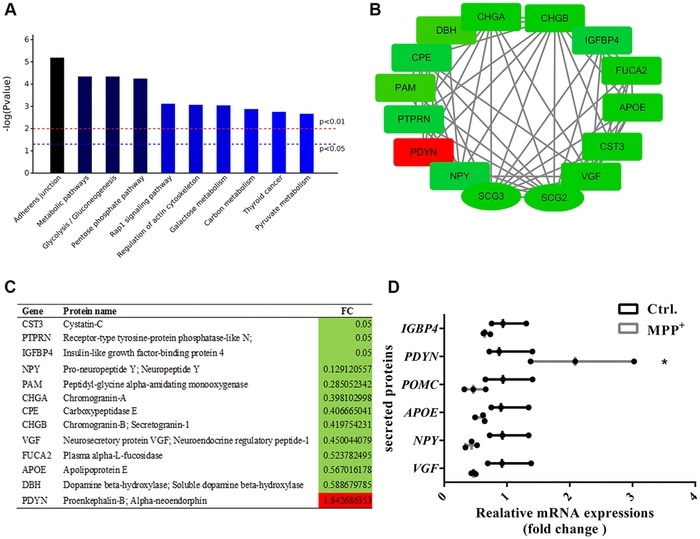
(A) The top 10 enriched KEGG pathways of the 56 secretory granules proteins and 140 secreted proteins. (B–C) Candidate proteins interacting with secretogranins in peptide processing in SGs indicated by the PPI network. (D) The expressions of some secretogranins-related candidate neuropeptides and neuroendocrine hormones are verified using real-time PCR. Two-tailed unpaired Student t-tests were performed between the control and treated groups. *Statistically significant with P < 0.05; Error bars are SD; N = 3. Image Credit: Xiaoni Zhan.
The article titled “Proteomic characterization of secretory granules in dopaminergic neurons indicates chromogranin/secretogranin-mediated protein processing impairment in Parkinson’s disease” was published in Aging-US.
Proteomic analyses of SGs purified from the dopaminergic SH-sy5Y cells under 1-methyl-4-phenylpyridinium treatments pinpointed 536 vital variably expressed proteins.
Protein-protein interaction examination of 56 secretory proteins and 140 secreted proteins indicates that the peptide processing intervened by chromogranin/secretogranin in SGs was extraordinarily compromised, followed by reduced candidate proteins and peptides neurosecretory protein, apolipoprotein E, neuropeptide Y, and a higher level of proenkephalin.
Parkinson’s disease (PD) is the second most common age-related neurodegenerative disorder.”
Dr Xiaoni Zhan, The China Medical University
The pathological characteristics of PD are increasing injury and loss of dopaminergic neurons in the substantia nigra pars compacta and the development of Lewy bodies and Lewy neurites. But the molecular mechanisms underlying PD are not known owing to the various factors concerned and their intricate interplay. The dysfunction of vesicle transport is implicated as an underlying mechanism of PD by emerging studies.
SGs symbolize the primary subcellular site for the biosynthesis, storage, and release of hormones and neuropeptides.
They are packed with cargo at the trans-Golgi network to develop primary vesicles, experience maturation steps as they migrate along cytoskeletal filaments, and eventually release the processed proteins responding to calcium signals. SGs’ secretory and processing machinery demands orchestrated actions of an incredible repertoire of ATP, lipids, enzymes, and cytoskeletal filaments.
Scg3 functions as a bridge between chromogranin A and the cargo aggregates. It also engages in early peptide processing by interacting with carboxypeptidase E in the vesicle membrane. In the earlier research works by the scientists, a toxin-induced by dopaminergic neuron model of PD was set up with paraquat.
The Zhan Research Team stated in their Aging-US Research Output that three of the 56 substantially differentially expressed proteins in secretory granules were also pinpointed or foretold in various omics research of PD. Calmodulin 3 codes for a family of proteins that attach calcium and function as an enzymatic co-factor.
However, CALM3 was foretold as one of the prioritizing genes for PD upon co-analysis of DEGs from the above microarray with the established PD-linked genes employing a network science approach.
Source:
Journal reference:
Wen, G., et al. (2021) Proteomic characterization of secretory granules in dopaminergic neurons indicates chromogranin/secretogranin-mediated protein processing impairment in Parkinson’s disease. Aging-US. doi.org/10.18632/aging.203415.