The human heart, upon aging, slowly loses its capability to repair itself after injury. Damage caused by injuries like heart attack and cardiac ischemia—linked to decreased oxygen levels in the heart—can result in below normal capacity of heart functioning, making it tough for the patients to carry out daily activities.
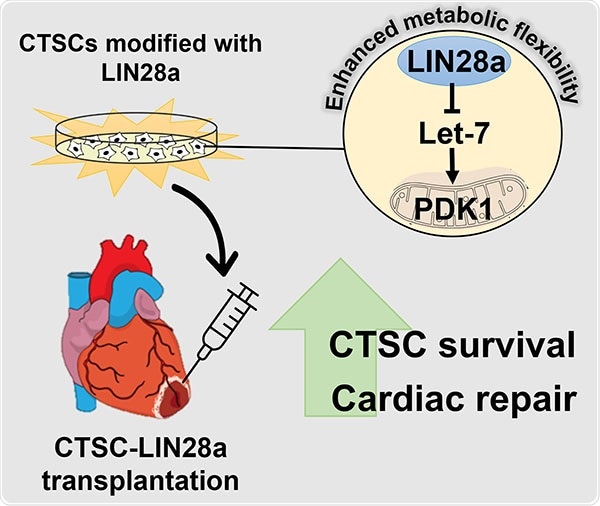
Image Credit: Temple University Health System.
Scientists recently switched on to stem cell-based therapies to augment heart repair after ischemic injury. These therapies replace dead heart tissue with new, functional tissue. Mostly less than 1% of the stem cells survive transplantation into the heart. This is mainly due to the cells’ incompetence to deal with the metabolic demands of the ischemic environment.
The current study carried out by the researchers from the Lewis Katz School of Medicine at Temple University demonstrated that, at least in mice, this problem could be overcome by the reintroduction of LIN28—a protein commonly expressed in the developing heart—into stem cells derived from adult heart tissue. LIN28 makes adult cardiac stem cells more metabolically flexible, highly enhancing their chances of survival.
LIN28 is very active in the developing heart, but not in the adult heart. We found that when we expressed LIN28 in cardiac stem cells from adult heart tissue, the adult cells were reprogrammed to have metabolic characteristics of young, developing heart cells. The process was essentially like reverse aging.”
Mohsin Khan, PhD, Study Senior Investigator and Assistant Professor, Cardiovascular Sciences, Cardiovascular Research Center, Lewis Katz School of Medicine
The observations were published in the journal Redox Biology.
The fetal heart is specifically adapted to function in low-oxygen conditions. The heart loses this low-oxygen tolerance post-natally, and by adulthood, it becomes highly sensitive to decreases in oxygen availability. The reason behind the shift is the changes in cellular metabolism, and current research works indicate that these metabolic differences in the heart help ascertain the fate of stem cells after transplantation.
Dr. Khan and co-workers in their research were fascinated in identifying whether the metabolic regulators expressed in the developing heart can provide a kind of metabolic flexibility to cardiac tissue-derived stem-like cells (CTSCs). CTSCs are found in both neonatal and adult heart tissue and express LIN28 alone in the developing heart. In adult heart tissue, CTSCs have regenerative potential and are mostly dormant.
The researchers initially reintroduced LIN28 expression in adult mouse CTSCs in vitro and examined the effects on signaling pathways involved in cellular metabolism, growth, and regeneration. They identified that LIN28 expression generated a powerful regenerative response, altering CTSCs to encourage growth and survival in response to oxidative stress.
The scientists associated the modifications to the Let7/PDK1 signaling pathway, which is known to control aerobic metabolism in cells. Transplantation of LIN28-expressing CTSCs into the heart in mice that endured heart attack resulted in substantial improvements in cardiac structure and function. The effects were mediated through the same pathways discovered in the in vitro assessments.
LIN28 modified energy production in CTSCs, leading to the secretion of many factors that are beneficial for heart cell survival. Overall, the cells took on a more youthful phenotype.”
Mohsin Khan, PhD, Study Senior Investigator and Assistant Professor, Cardiovascular Sciences, Cardiovascular Research Center, Lewis Katz School of Medicine
Dr. Khan intends to further translate the observations into a larger animal model and to identify whether human-derived cardiac stem cells can be reprogrammed by LIN28.
These studies could have important implications for how we approach stem cell therapy for heart disease in humans.”
Mohsin Khan, PhD, Study Senior Investigator and Assistant Professor, Cardiovascular Sciences, Cardiovascular Research Center, Lewis Katz School of Medicine
Source:
Journal reference:
Yuko, A. E., et al. (2021) LIN28a induced metabolic and redox regulation promotes cardiac cell survival in the heart after ischemic injury. Redox Biology. doi.org/10.1016/j.redox.2021.102162.