Electrostatic interaction is significant for numerous biological reactions—like protein-protein interaction, enzyme catalysis, protein-DNA/RNA interaction, and H+ transfer.
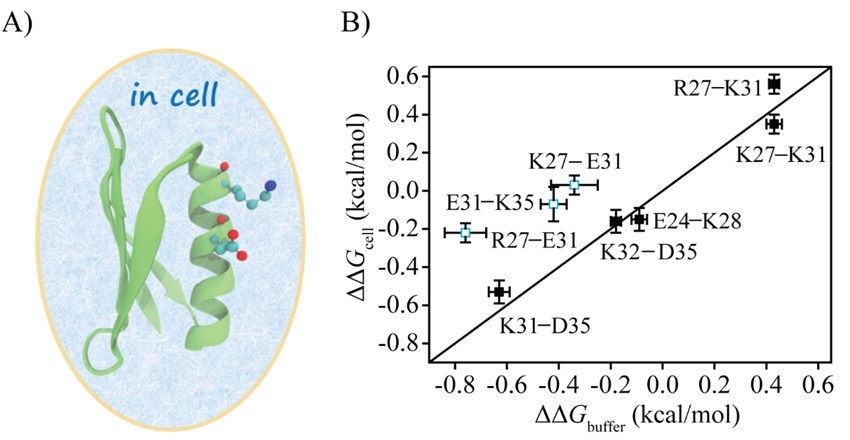
Image by Xiangfei Song, Mengting Wang, and Lishan Yao.
The majority of the proteins carry out their functions in cells and the complex cellular environment could disturb the electrostatic interactions of proteins. However, it is not known to date how the cellular environment modulates protein electrostatic interactions.
A group of scientists from the Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) of the Chinese Academy of Sciences (CAS) recently examined the protein electrostatic interactions in Escherichia coli cells using Nuclear Magnetic Resonance Spectroscopy.
The research was published on November 12th, 2021, in the Journal of the American Chemical Society.
The scientists used the double mutational cycle (DMC) method to directly measure the electrostatic interactions between protein charges in cells. They then compared the results with the ones measured in a buffer to comprehend the impact of the cellular environment on protein charge interactions.
The scientists introduced eight charge pairs in the protein GB3. Upon comparison to the charge pair electrostatic interactions in buffer, five charge pairs in cells showed no apparent changes, while three pairs had weakened interactions weakened by over 70%.
For the R27–E31 charge pair, the main perturbation was from the unfolded state, while for the E31–K35 charge pair, the main perturbation was from the folded state. E. coli cell lysate captured the weakening of electrostatic interaction of E31–K35 but not that of R27–E31. This shows that the lysate can imitate a specific type of interaction in cells.
Additional analyses revealed that the transfer of free energy was the reason for the electrostatic interaction modulation. The transfer-free energy of the unfolded state and that of the folded state can contribute to the cellular environmental impact on protein electrostatics.
However, those from the unfolded state were larger (more negative) than the ones from the folded state. This indicates that the cellular environment chose to interact with the unfolded state when compared to the folded state. These quinary interactions weaken the electrostatic interactions between some protein charge pairs.
Source:
Journal reference:
Song, X., et al. (2021) Quantifying Protein Electrostatic Interactions in Cells by Nuclear Magnetic Resonance Spectroscopy. Journal of the American Chemical Society. doi.org/10.1021/jacs.1c10154.