In this interview, we speak to researchers from the UC San Diego Center for Circadian Biology about their latest research that led to the development of a biological clock in a test tube.
Every part of our physiology is driven by a biological clock. What inspired your latest research into this biological clock known as a circadian clock?
It’s one thing to know the general outlines of how a biological process works, and another to understand it at the level of how the molecules interact. This very specific interaction of one molecule with another is how biology really works, and with the cyanobacterial clock in a test tube, we have the ability to gain those insights, because test tube conditions are highly defined and controllable, which is in contrast to the murkier goings-on inside live cells.
How vital is this circadian clock to the life of organisms? What would happen if this ‘clock’ was to break or stop functioning correctly?
Cyanobacteria with a broken clock are viable and grow fairly normally. However, if you mix them with cells that have normal clocks, they are rapidly lost from the population in a 24-h cycle of light and dark. That’s how evolution works.
Most genetic alterations make pretty subtle changes to biology, and the fittest organisms go on to survive and pass along their genes. In humans, there are a lot of situations where people’s clocks aren’t synchronized to the environment, such as shift work, jet lag, and a desynchronization associated with poor habits related to sleeping, eating, exercise, and light exposure. This out-of-balance situation isn’t deadly per se but contributes to a lot of illnesses.
Image Credit: Dr. Norbert Lange/Shutterstock.com
In your latest research, you assembled the circadian clock of cyanobacteria in a test tube. Can you describe how you did this?
The initial timing loop had been reconstituted in a test tube 15 years ago by Takao Kondo’s lab in Japan, so we already knew how to take 3 key proteins, KaiA, KaiB, and KaiC, and get them to set up a 24-h cycle of one biochemical event: the phosphorylation of the protein KaiC.
Our new work was an expansion of that system that allowed us to do two things: to measure more features of what is going on as time ticks by and to incorporate the components that get time information out of that loop and use it to control gene expression. The work created a whole new way of measuring the 24-h rhythm using fluorescence that can be monitored continuously for days without a person having to take samples manually and process them, and allowed the activities of different components to be followed, not just the phosphorylation of KaiC.
As a result, we could watch different components to see when each was interacting with another, add mutant versions to test our hypotheses about what surface of each protein interacts with another, and watch a protein bind to a DNA with a 24-h rhythm.
What were the aims for doing this and what did you discover?
We wanted to elucidate the steps that are making the cycle take 24 h, and see how information gets out of the timing loop.
We found that the same components that ferry the information out to control gene regulation also contribute to making the fundamental loop more robust, buffering it from changes in amounts of key components. They do it by mimicking the shapes of two of the key timing loop proteins, KaiA and KaiB, which allows them to compete with or recruit the protein each mimics to bind to a particular surface.
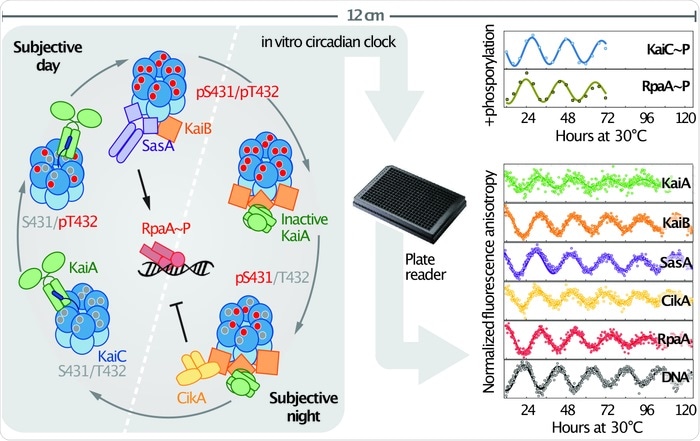
Image Credit: Andy LiWang
How are the molecular details of circadian rhythms in cyanobacteria similar to that of humans? What were the advantages of carrying out your research in a test tube (in vitro) compared to living cells (in vivo)?
The mechanics of the clocks in cyanobacteria are different than in humans, and yet their properties are the same in how they influence biology. The cyanobacterial system is really special in the sense that the clock mechanism is made up of only a few components, and they can carry out their functions without moving around to different compartments of cells. This is an aspect that lets us reconstitute the clock in a test tube.
But even better, we can also work in cells, so we get the molecular details from the very defined mixture in the tube, and then test those details in living cells. And, when we see clock activity in cells that we want to ask detailed questions about, we can see if our ideas check out in the test tube. What makes this system stand far apart from all others is being able to test hypotheses on oscillating clock reactions in test tubes and in real-time, and then see if they hold up inside live cells.
The ongoing COVID-19 pandemic has shown how fast scientific advancements can be made when researchers collaborate together. What role did collaboration play in your research?
This success is all about collaboration. The heads of the three labs all have different skill sets. We are each great at what we do, but what is easy for one of us would be really hard for another. Despite this gulf in expertise between us, we can communicate the significance of our findings, and the general outline of our methods to each other, which lets us toss the concept from one skill set to another to put together a more complete picture than any of us could do alone.
We also work very well together, sharing fresh ideas, reagents, and protocols. This collaboration built on friendship inspires us to identify and tackle challenging questions together.
Do you believe that your research will continue to help us gain a deeper understanding into how cells tell time as well as other important physiological features?
We continue to find surprising biochemical tricks at work in the clock, which are likely present in other biological processes and other types of organisms. Our relatively simple system alerts others to unexpected molecular events, so they know what to look for.
Indeed, the cyanobacterial system might seem quite dissimilar to eukaryotic clocks, but because all circadian clocks are composed of interacting proteins that need to generate ~24-h biochemical rhythms there are emerging conceptual similarities.
What are the next steps for your research?
Each of the collaborating labs has its own goals, but among them are figuring out how, at the molecular level, the clock is buffered from changes in temperature, and what happens when the clock resets (such as a laboratory-imposed change in time zone).
We also want to build more complexity into the test tube clock, because there are many more components that interact with it in cyanobacteria. Another goal is to encapsulate the test tube clock into cell-size vesicles to bring in vitro studies closer to the physiological setting.
Where can readers find more information?
Chavan AG, Swan JA, Heisler J, Sancar C, Ernst DC, Fang M, Palacios JG, Spangler RK, Bagshaw CR, Tripathi S, Crosby P, Golden SS, Partch CL, LiWang A. Reconstitution of an intact clock reveals mechanisms of circadian timekeeping. Science. 2021 Oct 8;374(6564):eabd4453. doi: 10.1126/science.abd4453. Epub 2021 Oct 8. PMID: 34618577.
About Dr. Susan Golden

Dr. Golden is Director of the UC San Diego Center for Circadian Biology, of which Drs. Partch and LiWang are also members. She is a Distinguished Professor of Molecular Biology at UC San Diego, an elected Fellow of the American Academy of Microbiology, and an elected Member of the National Academy of Sciences.
Her research focuses on the mechanism and biological consequences of circadian rhythms in cyanobacteria, and the development of these organisms for biotechnological purposes.
About Dr. Andy LiWang

Dr. Andy LiWang started studying the cyanobacterial circadian clock as an Assistant Professor at Texas A&M University after Dr. Golden introduced the system to him at a bar in 1999. He is now a Professor of Chemistry & Biochemistry at UC Merced and an elected Fellow of the American Academy of Microbiology.
His research focuses on the biochemical mechanism and structural biology of the cyanobacterial circadian clock.
About Dr. Carrie Partch

Dr. Carrie Partch is a Professor of Chemistry and Biochemistry at UC Santa Cruz. Her research focuses on the biochemical mechanisms and structural biology of the prokaryotic and eukaryotic circadian clocks.