Mitochondria are self-contained organelles that reside inside cells and are tasked with producing the chemical energy required to power vital tasks necessary for life and wellbeing. They have their own mini-chromosome and DNA.
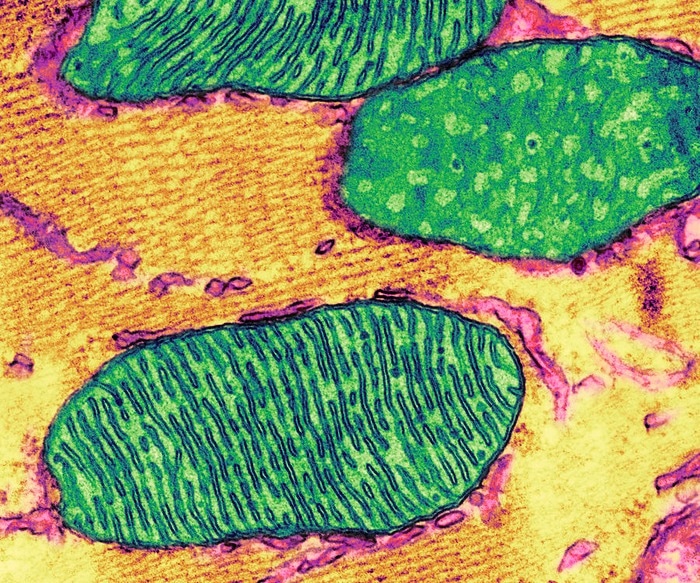 A colorized transmission electron micrograph depicts cellular mitochondria with its characteristic internal folds called cristae. Image Credit: Thomas Deerinck, National Center for Microscopy and Imaging Research, UC San Diego.
A colorized transmission electron micrograph depicts cellular mitochondria with its characteristic internal folds called cristae. Image Credit: Thomas Deerinck, National Center for Microscopy and Imaging Research, UC San Diego.
When under stress, injured, or malfunctioning, mitochondria release their oxidized and cleaved DNA (mtDNA) into the cytosol, the liquid that surrounds a cell and contains the organelles, and then into the circulation, causing inflammation. The levels of circulating oxidized mtDNA in autoimmune diseases including lupus and rheumatoid arthritis correspond with illness severity, flare-ups, and how well patients react to treatments.
If oxidized mtDNA is only a biomarker or indication of illness or something more—a crucial factor in disease pathology—is an issue that has plagued the profession and needs to be addressed.
Researchers from the University of California San Diego School of Medicine and colleagues from other institutions define the biochemical pathway that leads to the generation of oxidized mtDNA, how it is expelled by mitochondria, and how it triggers the intricate and destructive inflammatory response that follows in a new study that will be published in the Immunity journal on July 13th, 2022.
In addition to charting a new pathway responsible for the generation of inflammation-provoking fragments of oxidized mtDNA, this work opens the door to the development of new anti-inflammatory agents.”
Michael Karin, PhD, Study Senior Author and Distinguished Professor, Pharmacology and Pathology, University of California San Diego School of Medicine
One of the immediate responses of mitochondria to metabolic danger signals is for them to rapidly take up calcium ions from the cytosol, which causes the generation of reactive oxygen species that lead to the formation of oxidized mtDNA and the opening of pores in the mitochondrial membranes through which oxidized mtDNA escapes. Macrophages are a type of white blood cell that detects infections and tissue damage and mobilizes other immune system cells to respond.
However this oxidized mtDNA is large and before it can sneak through the mitochondrial pores, it needs to be chopped into smaller fragments. That job is carried out by an enzyme called FEN1.”
Hongxu Xian, PhD, Study First Author and Postdoctoral Scholar, Department of Pharmacology, University of California San Diego School of Medicine
Oxidized mtDNA fragments, after being cut by FEN1, reach the cytosol where they may connect with two distinct sensors, NLRP3 and cGAS. The inflammasome, a multi-protein complex that triggers inflammatory reactions, contains NLRP3. A tiny molecule that is produced by the enzyme cGAS serves as a chemical messenger to promote the synthesis of other cytokines, which are proteins that excite, attract, and promote the proliferation of immune cells.
Together, NLRP3 and cGAS promote inflammation, which has a tendency to run rampant in autoimmune illnesses and causes the immune system to target and damage healthy cells and tissues.
The latest research, according to Xian, emphasizes the crucial role FEN1 plays in stoking the “auto-inflammatory fire.” It is significant to note that Xian and associates have shown that FEN1 inhibitors block NLRP3 and cGAS signaling and so stop the start of the inflammatory process.
“This work is important not only because it can explain the origin and pathogenesis of common rheumatic diseases, but it can also lead to the development of new biomarkers and treatments for lupus and arthritis.” Stated Monica Guma, MD, PhD, Associate Professor from the UC San Diego School of Medicine and rheumatologist at UC San Diego Health, who was not involved in the research.
Source:
Journal reference:
Xian, H., et al. (2022) Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. doi.org/10.1016/j.immuni.2022.06.007.