The world’s first CRISPR-Cas9 genetic editing-based strategy to regulate inheritance in mammals was declared nearly three years ago by researchers at the University of California, San Diego.
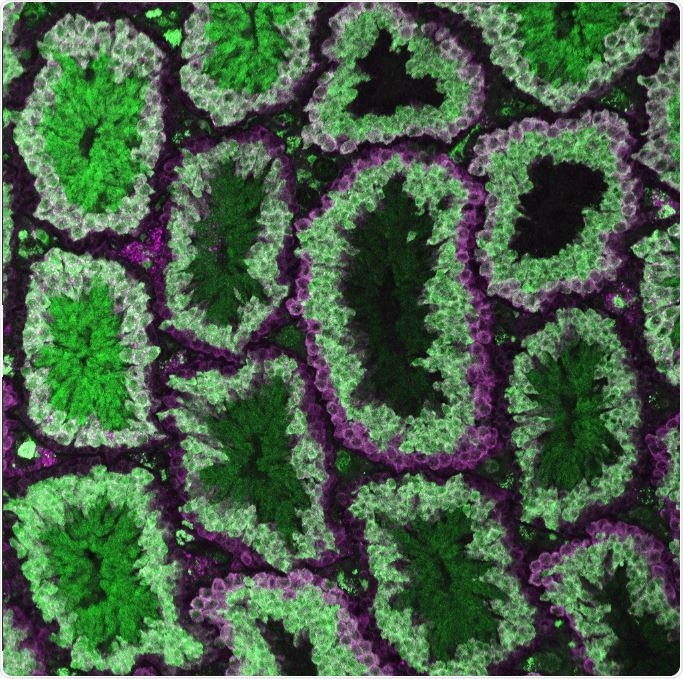
Researchers discovered that CRISPR-Cas9 genetic editing during meiosis was key to controlling allelic inheritance in male and female mice. The image shows male germline stem cells (magenta only) and Cas9 expression in meiotic spermatocytes marked by the overlap of magenta and green. Image Credit: Cooper Lab, University of California–San Diego.
Hannah Grunwald, a graduate student at UC San Diego, and Associate Professor Kimberly Cooper performed research that used “active genetics” editing, a technique created at UC San Diego, to impact the inheritance of genes in mice.
This allows researchers to select which copy of a gene is carried down from generation to generation, which might be useful in a number of biomedical and environmental applications. The study was successful in female mice but not in male mice, owing to changes in the timing of major events during the reproductive cell division process known as meiosis in females and males.
Grunwald, Cooper, and colleagues, headed by graduate student Alexander Weitzel, have succeeded in generating CRISPR-Cas9 inheritance control in male mice by adjusting the gene-editing window to more precisely match the time of meiosis in both sexes. Their findings were published on December 23rd, 2021, in the journal PLOS Biology.
The development increases the probability that scientists will be able to apply genetic editing for novel laboratory models in a variety of research endeavors, ranging from human disease research to therapeutic drug discovery to invasive species elimination.
For these gene conversion strategies to work in any context—in the lab or in wild populations—you need the mechanism of gene conversion to work in both males and females. It seems as though the reason this process was previously working in females is because we were closer to the female meiotic window. Now that we’ve moved Cas9 expression to within the meiotic window in males, it works in them too.”
Kimberly L. Cooper, Associate Professor, Section of Cell and Developmental Biology, Division of Biological Sciences, University of California–San Diego
The researchers employed a “CopyCat,” an active genetic DNA element that replicates genetic information in mice, as before. In the current experiments, the researchers used the regulatory DNA of Spo11, a gene known to be involved in meiosis in both males and females, to control the production of the Cas9 protein, which breaks DNA.
Since timing is so important, hitting that meiosis sweet spot was important for male gene conversion.”
Kimberly L. Cooper, Associate Professor, Section of Cell and Developmental Biology, Division of Biological Sciences, University of California–San Diego
As earlier, there were several limitations to make the new method succeed. The gene conversion process became less successful in order to arrive in the male timing window while still being inside the female timing window, most likely due to a reduced level of Cas9 expression. Cooper’s laboratory is now exploring this problem.
“While showing that CRISPR-Cas9-mediated gene conversion is possible in both male and female mouse germlines, our work reveals nuances that differ from insects and that must be considered for further refinement and implementation in rodents,” the scientists say in the study.
In a recent study, Grunwald, Weitzel, and Cooper have published a form of instruction manual for other laboratories interested in mammal gene conversion.
The researchers discuss their CRISPR-Cas9 gene conversion system and its potential for usage in the laboratory and in wild rodent populations were published as an article in Nature Protocols on December 23rd, 2021. They outline the technical challenges that must be addressed before scientists may apply such methods in any environment. The scientists suggest that expanding this method would increase laboratory efficiency, which would be beneficial on several levels.
The authors add, “If successfully implemented in the laboratory, CRISPR-Cas9-mediated gene conversion promises to save money, time and animal lives, while simultaneously expanding our ability to investigate some of the most complex developmental questions and most prevalent human diseases.”
Source:
Journal reference:
Weitzel, A. J., et al. (2021) Meiotic Cas9 expression mediates gene conversion in the male and female mouse germline. PLoS Biology. doi.org/10.1371/journal.pbio.3001478.