According to recent research carried out by researchers from the Weill Cornell Medicine, a gene mutation associated with Alzheimer’s disease changes a signaling pathway in specific immune cells of individuals with the disease. The researchers also discovered that hindering the pathway—with a drug being tested in cancer clinical trials—safeguards against numerous features of the condition in a preclinical model.
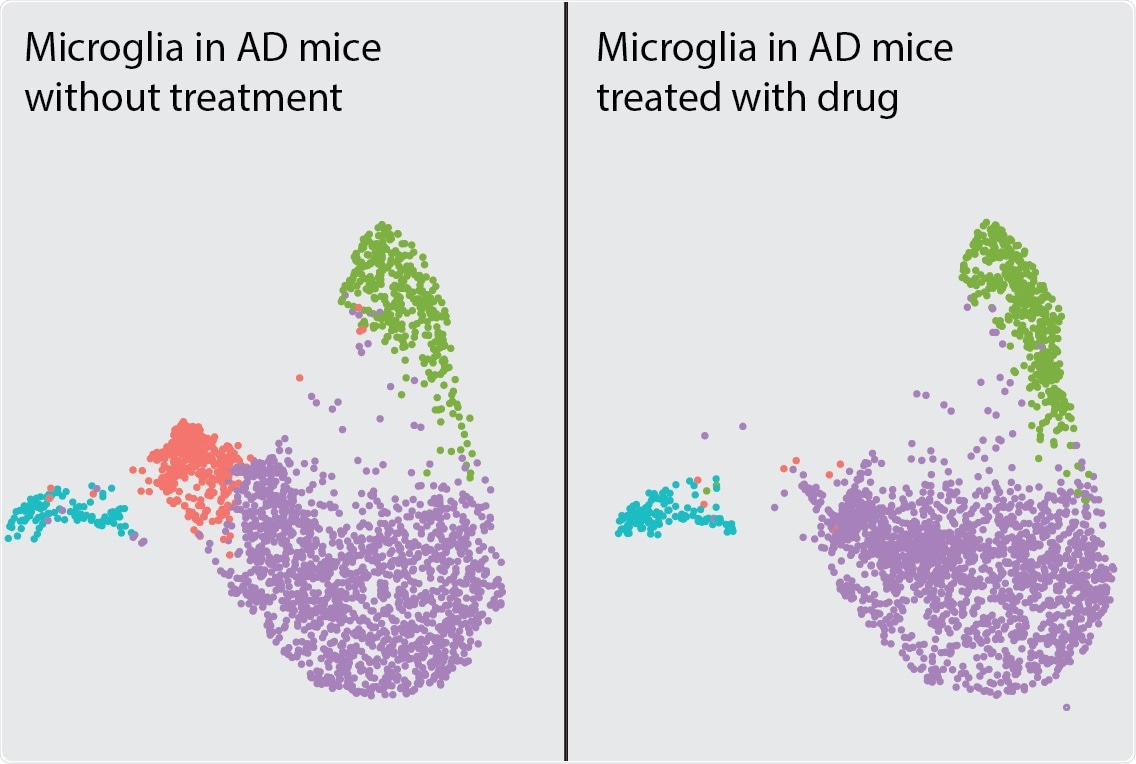
Single cell RNA-seq analysis of microglial response in a mouse model of Alzheimer’s disease. Treatment with experimental drug MK2206 depleted a subcluster of disease-associated microglia (pink) and protected against synaptic loss (not pictured). Each dot depicts one microglial cell and different colors depict different microglial states. Image Credit: Dr Li Gan.
The observations can lead to novel strategies to impede the development of Alzheimer’s disease or delay its progression.
The research concentrated on microglia—immune cells of the central nervous system that responds first when something goes wrong in the brain. Several pieces of research have found numerous genetic variants associated with Alzheimer’s disease that is greatly expressed in microglia, offering convincing evidence that the changes within these cells might perform a role in the inception of the disease and its progression.
The research was published on December 1st, 2021 in the Science Translational Medicine journal.
Microglia are guardians of the brain under healthy conditions but can turn detrimental in disease conditions. Our goal is to identify how they become toxic and contribute to Alzheimer’s disease pathogenesis and whether we can identify immune modulators to reverse the toxicity without diminishing their normal protective function.”
Dr. Li Gan, Study Senior Author and Director, Helen and Robert Appel Alzheimer’s Disease Research Institute, Weill Cornell Medicine
Dr. Li Gan is also the Burton P. and Judith B. Resnick Distinguished Professor in Neurodegenerative Diseases in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
Alzheimer’s disease is the most frequent neurodegenerative disease in aging. It affects around 46 million people globally. Theories indicate numerous potential reasons like age-related changes in the brain, environmental, genetic, and lifestyle factors. These lead to the aggregation of toxic proteins in the brain—and recent evidence suggests immune system changes—that result in the loss of neurons and their connections.
To analyze how the brain’s immune cells might contribute to Alzheimer’s disease, Dr Gan and her co-workers initially established the molecule fingerprint of individual microglia in the brains of patients with Alzheimer’s disease. These patients carry a mutation in the TREM2 gene that increases the individual’s risk for advancing Alzheimer’s disease.
TREM2 is a receptor mostly expressed by microglia in the brain, and amongst other functions, it signals through an enzyme called AKT to control metabolism and inflammation.
The researchers later established a mouse model by bringing together two strains; one that has the AD-linked mutation in the TREM2 gene and another that showcases Tau aggregates—a key pathological hallmark in Alzheimer's brains. Both mice and patients with the mutation showed memory-related shortcomings, and their microglia expressed increased levels of inflammatory molecules and showed an overactive AKT signaling pathway.
In mice, when the AKT was inhibited with a drug named MK-2206 reversed the inflammatory properties of microglia and safeguarded against synaptic toxicity—a kind of damage to the brain’s neurons that is characteristic of Alzheimer’s disease.
As AKT signaling also contributes to the pathogenesis of various types of cancer, MK-2206 is recently being analyzed in multiple cancer clinical trials. Hence, the safety of the drug is already under examination.
We identified a small molecule compound that has been tested in patients with cancer, readily enters the brain, potently modulates the brain’s immune responses, and protects against synaptic loss in animal models of Alzheimer’s disease. Our findings support further study of this compound as a potential therapy for Alzheimer’s disease.”
Dr. Li Gan, Study Senior Author and Director, Helen and Robert Appel Alzheimer’s Disease Research Institute, Weill Cornell Medicine
Source:
Journal reference:
Sayed, F. A., et al. (2021) AD-linked R47H-TREM2 mutation induces disease-enhancing microglial states via AKT hyperactivation. Science Translational Medicine. doi.org/10.1126/scitranslmed.abe3947.