Case reports of younger COVID-19 patients developing Parkinson’s disease within weeks of contracting the virus led researchers to investigate a possible link between the two conditions.
The SARS-CoV-2 N-protein can interact with α-synuclein in the test tube and help it form amyloid fibrils, a hallmark of Parkinson’s disease. Image Credit: Adapted from ACS Chemical Neuroscience, 2021.
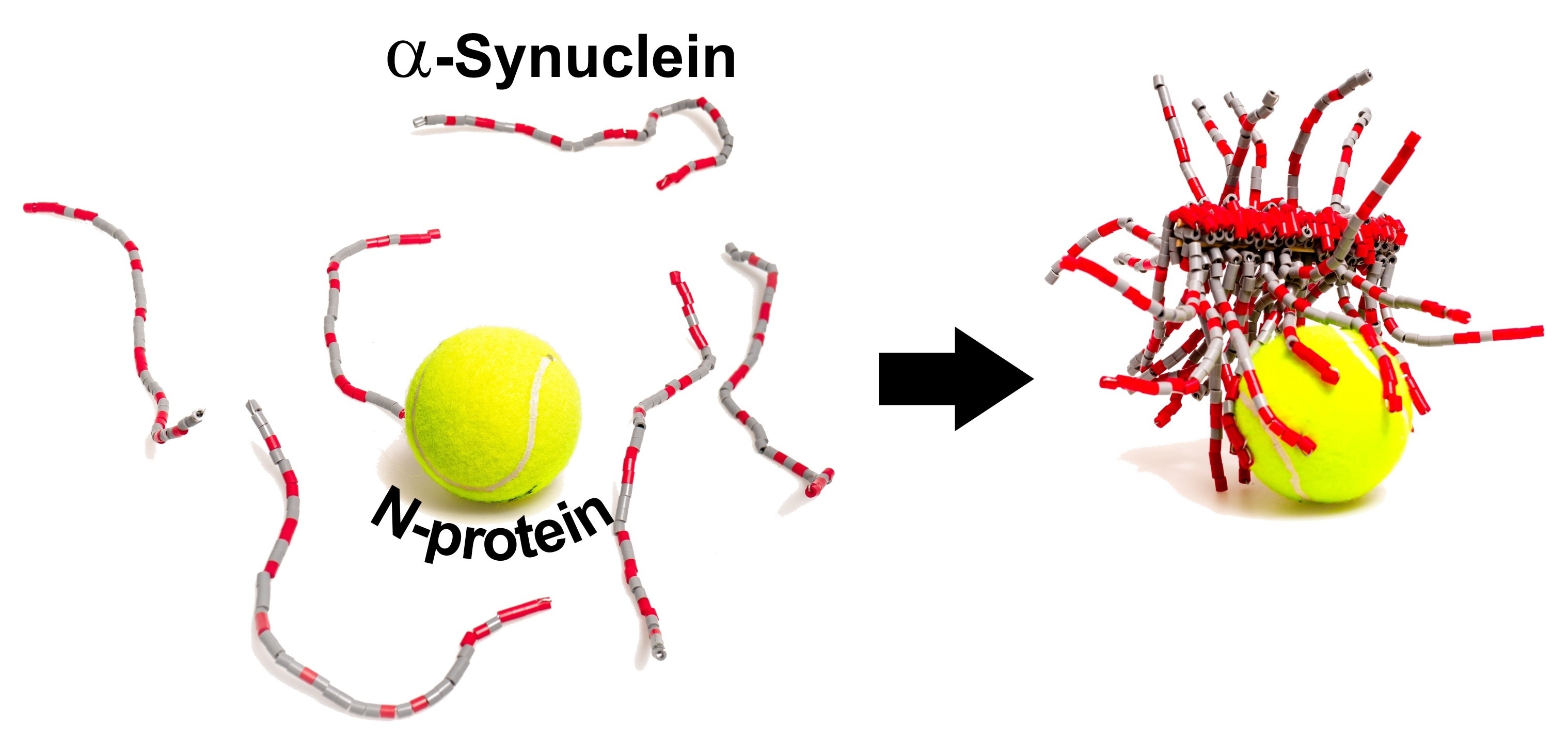
Scientists recently demonstrated that, in the test tube, the SARS-CoV-2 N-protein interacts with a neuronal protein known as α-synuclein and boosts the development of amyloid fibrils—the pathological protein bundles characteristic of Parkinson’s disease. The research was published in the ACS Chemical Neuroscience journal.
Along with causing respiratory symptoms, SARS-CoV-2 can induce neurological problems, like headaches, loss of smell, and “brain fog.” However, it is still controversial if these symptoms are caused by the virus penetrating the brain, or if the symptoms are due to chemical signals released in the brain by the immune system as a response to the virus.
In Parkinson’s disease, a protein known as α-synuclein develops abnormal amyloid fibrils, which leads to the death of dopamine-producing neurons in the brain. Intriguingly, in Parkinson’s disease, loss of smell is a usual premotor symptom.
This fact along with case reports of Parkinson’s in COVID-19 patients led Christian Blum, Mireille Claessens, and coworkers to wonder if the protein components of SARS-CoV-2 could induce the accumulation of α-synuclein into amyloid.
The researchers analyzed the two abundant proteins of the virus—the spike (S-) protein that facilitates the entry of SARS-CoV-2 into the cells and the nucleocapsid (N-) protein that encapsulates the RNA genome within the virus.
The researchers performed test tube experiments where they utilized a fluorescent probe that attaches amyloid fibrils to demonstrate that—in the absence of the coronavirus proteins—α-synuclein needed over 240 hours to aggregate into fibrils. When the S-protein was added, the researchers found no effects, however, the N-protein reduced the aggregation time to less than 24 hours.
The researchers carried out other experiments to demonstrate that the N- and α-synuclein proteins interact directly. The interaction is partly due to their opposite electrostatic charges, with around 3–4 copies of α-synuclein linked to each N-protein.
The scientists later injected the fluorescently labeled α-synuclein and N-protein into a cell model of Parkinson’s disease which contained the same concentration of N-protein that would be seen inside a SARS-CoV-2-infected cell.
Twice as many cells died when injected with both proteins compared to control cells with only α-synuclein injected. It was also found that the distribution of α-synuclein was changed in cells co-injected with both proteins.
The researchers observed elongated structures even though they could not confirm that they were amyloid. The researchers state that they are unsure if these interactions take place inside neurons of the human brain, however, if that is the case, it could illustrate the possible connection between COVID-19 infection and Parkinson’s disease.
Source:
Journal reference:
Semerdzhiev, S. A., et al. (2021) Interactions between SARS-CoV-2 N-Protein and α-Synuclein Accelerate Amyloid Formation. ACS Chemical Neuroscience. doi.org/10.1021/acschemneuro.1c00666.