An individual’s breathing increases greatly when they are hurt or anxious—for instance, when terrified by a threatening noise, the breathing quickens or when individuals bang their elbow they pant in pain. However, the reason for this was not perceived earlier. Recently, a group of Salk scientists unraveled a neural network in the brain that coordinates breathing rhythm with feelings of fear and pain.
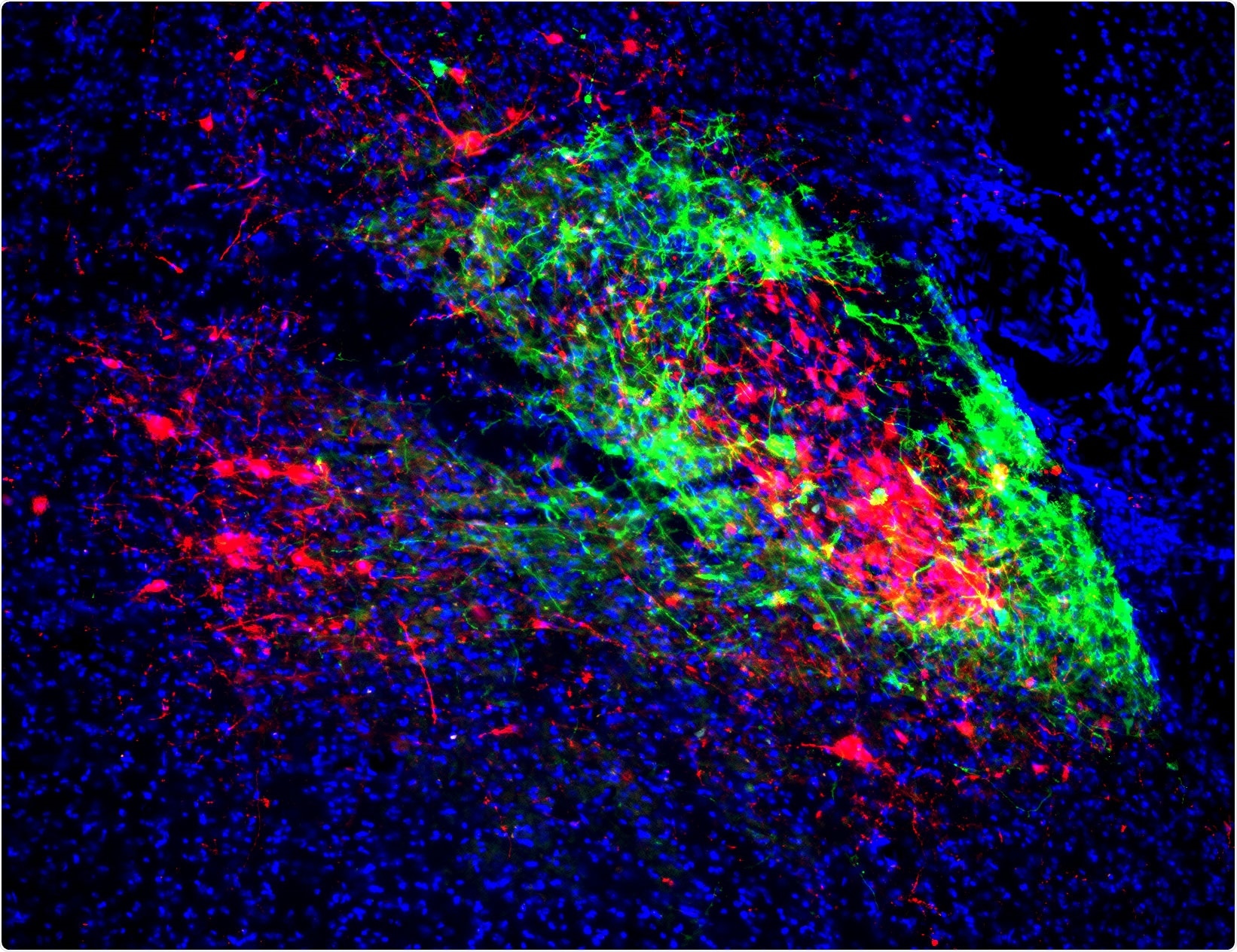
Shell neurons (green) that project to the breathing center and core neurons (red) that project to the pain/emotion center. Image Credit: Salk Institute.
The observations of the research in addition to the fields of pain management, philosophical investigations into the nature of pain, and psychological theories of anxiety can lead to the generation of an analgesic that inhibits opioid-induced respiratory depression (OIRD)—disrupted breathing that results in overdose deaths.
The scientists from Salk concentrated on a group of neurons in the brainstem known as the lateral parabrachial nucleus, organized in a core-shell configuration. They observed that the neurons in the shell project to the pre-Bötzinger complex, a region that creates breathing rhythm, and that the neurons in the core project to the amygdala, an area of the brain that handles fear and the emotional experience of pain.
The shell and core neurons impact each other depending on the inputs from these areas, resulting in faster breaths when experiencing anxiety or pain. The research was published on December 17th, 2021, in the journal Neuron.
We are the first group to demonstrate how the lateral parabrachial nucleus coordinates breathing and pain. By understanding the circuits in this brain region, we may be able to tease apart breathing regulation and pain regulation to develop a medication that inhibits feelings of pain without repressing breathing, like OIRD.”
Sung Han, Study Senior Author and Assistant Professor, Clayton Foundation Laboratories for Peptide Biology, The Salk Institute
In OIRD, opioids repress breathing and also pain—the leading cause of death from opioids. In prior research, Han’s laboratory revealed that opiates similar to morphine suppress breathing by inducing specific receptors known as mu opioid receptors (MOR), resulting in the inhibition of neurons that express them.
The researchers also revealed that reactivating the cells expressing MOR can reverse OIRD. The current research puts forth additional measures for obstructing OIRD by inhibiting neurons in the region’s core (mitigating anxiety/fear) while stimulating similar neurons in the shell (supporting breathing).
To demonstrate the coordination of these neurons with emotions and pain, the scientists initially utilized chemical agents and light to substantiate that manipulating the MOR-expressing neurons found in the lateral parabrachial nucleus changes the rate of breathing in mice.
The researchers later used fluorescent tracers to map the outputs and inputs to the MOR-expressing neurons. The observations revealed that neurons agglomerated in the core of the region project to the central amygdala, but the neurons agglomerated in the surrounding shell project to the pre-Bötzinger complex.
The electrophysiological recordings of one population while stimulating the other population showed that some of those subpopulations were connected reciprocally, with a stimulative network between them. The signals of pain and fear were connected with breathing rhythms through this network.
We have found very intricate circuits involving upstream and downstream input to these neurons. By uncovering this circuit mechanism, we can better explain why breathing can often be coordinated with pain and anxiety.”
Shijia Liu, Study First Author and Graduate Student, The Salk Institute
Shijia Liu is a graduate student in Han’s laboratory.
Han is keen to witness the group’s discovery have a translational application.
The biggest problem these days is that opioids reduce pain but also reduce breathing, so people die. By understanding those two mechanisms in our research, maybe we can manipulate certain populations of neurons by pharmacological intervention so that we can control pain without changing the breathing.”
Sung Han, Study Senior Author and Assistant Professor, Clayton Foundation Laboratories for Peptide Biology, The Salk Institute
Han is the holder of the Pioneer Fund Development Chair.
At present Han’s team is absorbed in the genetic analyses of the shell and core population to pinpoint functional markers that particularly control breathing or pain.
Source:
Journal reference:
Liu, S., et al. (2021) Divergent brainstem opioidergic pathways that coordinate breathing with pain and emotions. Neuron. doi.org/10.1016/j.neuron.2021.11.029.