The respiratory chain performs a major role in the cell’s energy metabolism. It is localized in mitochondria—the power plants of the cell.
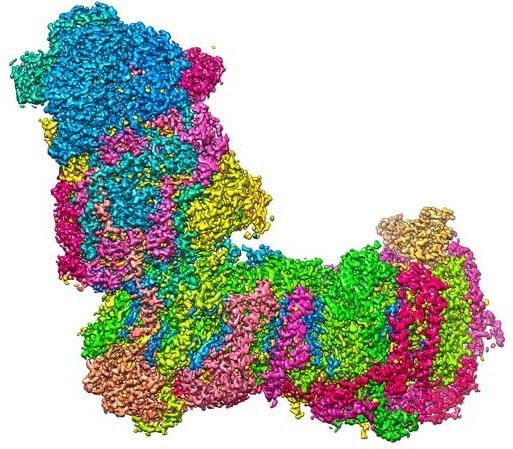
A bit like a boot: The L-shaped structure of mitochondrial complex I at a resolution of 2.1 Å (0.00000021 mm), captured with a cryo-electron microscope. Image Credit: Janet Vonck, MPI of Biophysics.
In recent research, scientists from Goethe University, the Max Planck Institute of Biophysics, and the University of Helsinki have identified the high-resolution structure of a major component of the respiratory chain—mitochondrial complex I—and simulated its dynamics on the computer.
The observations back primary research and also improve the knowledge of some neurodegenerative and neuromuscular diseases associated with mitochondrial dysfunction.
A constant supply of energy is necessary for all significant processes and in cells, ATP—the chemically “charged” molecule—is the major provider of energy. The ATP power packs are produced, amongst others, in specialized small organs (“organelles”) of the cell called the mitochondria.
In mitochondria, the protein complexes involved in the respiratory chain pump hydrogen ions (protons containing a positive charge) from one side of the inner mitochondrial membrane to the other (“uphill”).
This results in a chemical concentration gradient and an electrical voltage. The protons “flow downhill” along this electrochemical gradient through a kind of turbine that produces useful energy for the cell in the form of ATP.
One among the proton that pumps in the initial step of the process is a huge, L-shaped biomolecule known as mitochondrial complex I (complex I for short) whose horizontal arm is fastened to the membrane.
The vertical arm anchors the electron carrier molecule NADH, produced during the metabolic breakdown of sugar and other nutrients. Complex I catalyzes the electron transfer from NADH to ubiquinone (Q10), and the energy discharged during this reaction is utilized to drive the proton pump.
The scientist from Goethe University and the Max Planck Institute of Biophysics in Frankfurt employed cryo-electron microscopy to analyze the 3D structure of complex I at high resolution. The scientist demonstrated that the water molecules in the protein structure perform a vital role in establishing proton translocation pathways.
The high-resolution structural data allowed co-workers from the University of Helsinki to carry out extensive computer simulations which revealed the dynamics of the protein structure during its catalytic cycle.
Our study delivers new insights into how a molecular machine in biological energy conversion works.”
Dr Janet Vonck, Max Planck Institute of Biophysics
“This knowledge can contribute to a better understanding of certain mitochondrial diseases, such as loss of vision in Leber hereditary optic neuropathy,” states Professor Volker Zickermann from the Institute of Biochemistry II at Goethe University.
Source:
Journal reference:
Parey, K., et al. (2021) High-resolution structure and dynamics of mitochondrial complex I—Insights into the proton pumping mechanism. Science Advances. doi.org/10.1126/sciadv.abj3221.