Researchers recently identified a new role of the protein tBID, which was to date linked to the regulatory role in cancer and cell death. But tBID also directly mediates apoptosis (controlled cell death). This observation uncovers a novel approach to cancer therapy. The study was published in The EMBO Journal.
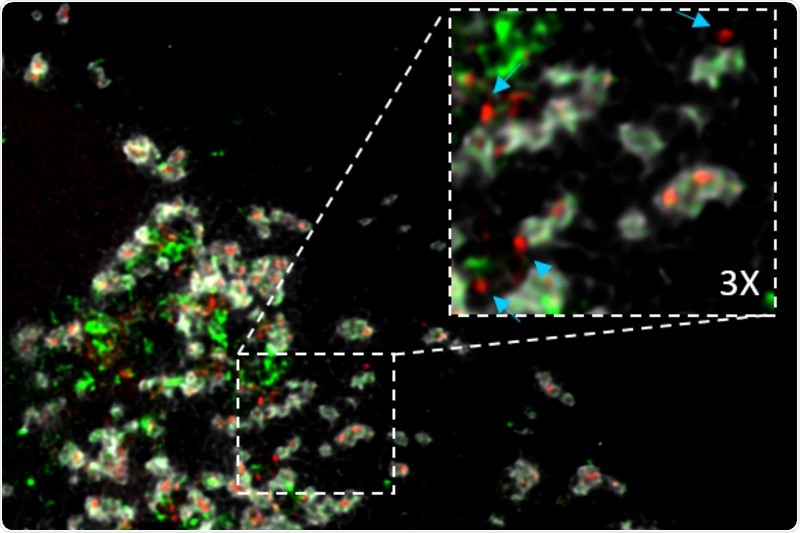
The Apoptosis related protein tBID induces mitochondrial DNA release in cells where the natural killers BAX and BAK are absent, replacing their function. Mitochondria appears in white, mitochondrial DNA in red, and the protein tBID in green. Cyan arrows show mitochondrial DNA released from the mitochondria. Of note, mitochondrial DNA release can activate inflammation pathways and immune system. Image Credit: ©Hektor Flores-Romero.
The protein tBID induces apoptosis (programmed cell death) by enticing damage in the mitochondria, the energy suppliers of the cells. Apoptosis is vital for retaining tissue balance. Moreover, it also plays a vital role as a defense mechanism and in the removal of redundant or damaged cells in the body.
Impaired apoptosis is associated with numerous human diseases, from cancer and autoimmune diseases to neurological disorders and heart failure.
The findings by Professor Dr Ana Garcia-Saez and her associates and coworkers at the University of Cologne’s CECAD Cluster of Excellence for Aging Research that tBID, earlier considered to be a signal transducer, also perform apoptosis uncovers novel therapies to treat malignant cells like cancer cells.
The protein tBID belongs to the family of BCL-2 proteins, vital for the self-determined apoptosis of cells and tissue balance. The overlapping functions of this protein family make it challenging to investigate the proteins individually. Garcia-Saez and her group characterized the roles carried out by the different family members.
With the help of cell lines short of the BCL-2 proteins, the researchers determined the function of tBID. They also used advanced microscopy (STED, confocal, and electron microscopy) to examine at length the impacts of tBID at the mitochondrial membrane in the cell. tBID activation helped fight cellular infection caused due to the bacterium Shigella flexneri and also destroy blood cancer cells (leukemia).
Moreover, the scientists demonstrated that apoptosis induced by tBID is independent of other known apoptosis induction pathways.
For us, it was amazing to see that proteins which are quite well known still surprise us after four decades. The realization that a protein that was considered a signal transducer for twenty years has an effector function under certain conditions is mind-blowing.”
Dr Ana Garcia-Saez, Professor, University of Cologne
This currently identified function of tBID could in the future become helpful in medicine.
For example, activating tBID could induce apoptosis when other known apoptosis signaling pathways fail or lack the proteins that carry it out. Activating tBID could also help with Shigella infections, where the proteins that usually induce apoptosis are not activated.”
Dr Ana Garcia-Saez, Professor, University of Cologne
The work for this research started at the IFIB (Interfaculty Institute of Biochemistry) in Tübingen. It was later developed at the CECAD Research Center, Institute for Genetics, in Garcia-Saez’s core laboratory, along with the labs of Professor Dr Hamid Kashkar and Dr Lukas Frenzel and external associates from the University of Nebraska and the lab of Andreas Villunger (University of Innsbruck and CeMM Research Center, Austria).
Source:
Journal reference:
Flores-Romero, H., et al. (2021) BCL-2-family protein tBID can act as a BAX-like effector of apoptosis. The EMBO Journal. doi.org/10.15252/embj.2021108690.