Autism spectrum disorders are characterized by a wide range of behavioral issues. However, one of the biggest problems for scientists and physicians is the heterogeneity of their symptoms.
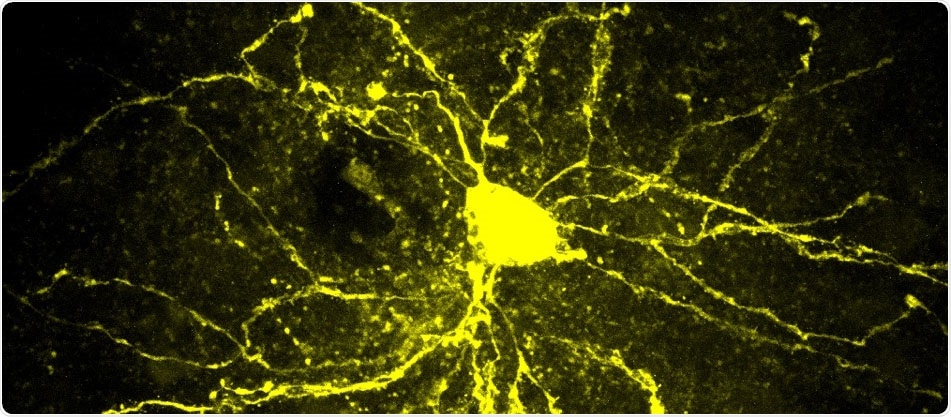
Medium Spiny Neuron located in the nucleus accumbens, one of the neural networks of the reward system. Image Credit: Camilla Bellone–Université De Genève.
While a link between the inflammatory process and autism has been long suspected, a team from the University of Geneva (UNIGE), Switzerland, as part of the Synapsy National Centre of Competence in Research, has for the first time comprehended how a change in cell environment triggers the onset of autistic symptoms in mice with genetic vulnerability. Indeed, a huge inflammatory response causes an imbalance in the expression of a number of genes.
Hyperexcitability of reward system neurons occurs as a result of an immunological response to the injection of a pharmaceutical substance. These results are the first to show that genes and the environment interact in the social dysfunctions that are common in autistic disorders, and they will be published in the journal Molecular Psychiatry.
The reward system has a role in the social interaction deficit in autistic mice, according to a study team headed by Camilla Bellone, a professor in the Department of Basic Neurosciences at the UNIGE Faculty of Medicine and director of the Synapsy National Centre of Competence in Research. Indeed, the activation of the neural networks that make up the reward system is directly connected to the desire that pushes people to communicate with their peers.
But what are the molecular and cellular pathways that cause social interaction deficits? The scientists analyzed so-called heterozygous mice, which have a deletion of only one of the two copies of the SHANK3 gene but do not display social behavioral abnormalities, to better understand this mechanism and thereby interpret how the symptoms emerge. This is one of the most prevalent monogenic causes of autism, accounting for 1–2% of all cases.
Humans carry a mutation in only one of the two copies of SHANK3, a gene that is essential for the functioning of synapses and communication between neurons. In animal models of the disease, however, mutation of a single copy of SHANK3 only slightly affects the behavior of mice, which explains why the behavioral phenotypes observed are not homogeneous”.
Camilla Bellone, Professor, Department of Basic Neuroscience, Faculty of Medicine, University of Geneva
The role of neuronal hyperexcitability
To find the additional genes whose expression was altered, the researchers first suppressed the expression of SHANK3 in the reward system’s neuronal networks. Several genes associated with the inflammatory system were discovered, including Trpv4, which is also important in the functioning of neuronal communication channels. Furthermore, scientists were able to restore normal social behavior by suppressing Trpv4.
Camilla Bellone adds, “By inducing massive inflammation, we observed an overexpression of Trpv4, which then led to a neuronal hyperexcitability concomitant to the onset of social avoidance behaviors that our mice did not exhibit until now.”
This provides evidence that autistic disorders are indeed the result of an interaction between a genetic susceptibility and an external trigger - in this case, massive inflammation. Neuronal hyperexcitability disrupts communication channels, thereby altering the brain circuits governing social behavior.”
Camilla Bellone, Professor, Department of Basic Neuroscience, Faculty of Medicine, University of Geneva
This would also explain why the same genetic predisposition may result in a wide range of symptoms of varying intensity, depending on the environmental conditions encountered and the sort of inflammation they cause.
Irreversible damage during development?
The inflammation was produced in adult animals in this investigation. The associated social deficit was not only reversible, but also vanished on its own after a few days.
We now need to replicate our research during the critical phases of neurodevelopment—i.e., during gestation and immediately after birth—in order to observe the impact of hyperexcitability on the developing neural networks. This could damage the construction of neural networks beyond repairs.”
Camilla Bellone, Professor, Department of Basic Neuroscience, Faculty of Medicine, University of Geneva
This research establishes a clear causal link between inflammation and the onset of behavioral symptoms in the context of genetic vulnerability, and it emphasizes the relevance of environmental variables, which have previously been overlooked. It also emphasizes the need to deepen our understanding of the processes behind autism diseases in order to intervene effectively.
Indeed, based on the gene-environment interactions and inflammatory processes unique to each patient, a therapy might be developed to match the cellular and molecular changes in the brain circuits.
Source:
Journal reference:
Tzanoulinou, S., et al. (2022) Inhibition of Trpv4 rescues circuit and social deficits unmasked by acute inflammatory response in a Shank3 mouse model of Autism. Molecular Psychiatry. doi.org/10.1038/s41380-021-01427-0.