Single-cell RNA sequencing—or shortly scRNA-seq—is an approach that lets researchers to study the gene expression in an individual cell within a mixed population—the way how all cells live virtually in the tissues of the body.
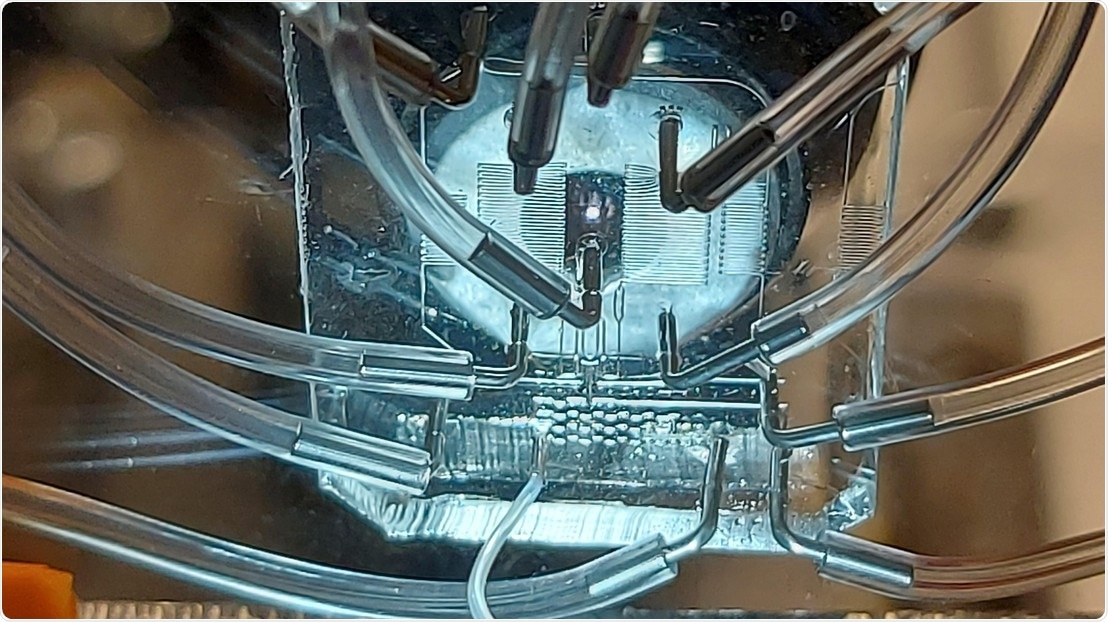
Close-up of the microfluidics enabling deterministic co-encapsulation of single cells at outstanding efficiencies. Image Credit: Joern Pezoldt (EPFL)
Part of a bigger family of “single-cell sequencing” methods, scRNA-seq includes acquiring the RNA of a single cell and, further multiple molecular conversion reactions, thereby sequencing it. As RNA is the transitional step from gene (DNA) to protein, it offers an indication about which genes in that specific cell are active and inactive.
As scRNA-seq acquired all genes activities in the cell genome—thousands of genes at the same time—it has become the classic standard for outlining phenotypes and cell states. This data type can show rare cell kinds inside a cell population, even those which were never been witnessed before.
Cost and efficiency
However, scRNA-seq is not just limited, as a tool for basic cell biology. It has been extensively adopted in pharmacological and medical research because it is able to identify which cells are dividing in a tissue actively, or which cells are responding to a specific drug or therapy.
These single-cell approaches have transformed our ability to resolve cellular properties across systems. The problem is that they are currently tailored toward large cell inputs.”
Bart Deplancke, Professor, School of Life Sciences, École Polytechnique Fédérale de Lausanne
As scRNA-seq methods need more than a thousand cells for a significant measurement, this is not a trivial issue.
This renders them inefficient and costly when processing small, individual samples such as small tissues or patient biopsies, which tends to be resolved by loading bulk samples, yielding confounded mosaic cell population read-outs.”
Dr Johannes Bues, Researcher, École Polytechnique Fédérale de Lausanne
Dr Bues is a researcher in Deplancke’s Group.
The DisCo solution
Now, Bues, with Marjan Biočanin and Joern Pezoldt, has developed a new approach that enables scRNA-seq to effectively process samples that have fewer cells. The method is named “DisCo” for “deterministic, mRNA-capture bead and cellco-encapsulation dropleting system.” The study was published in the journal Nature Methods.
DisCo employs machine-vision to actively identify cells and acquire them in droplets of beads and oil, unlike conventional single-cell methods that depend on passive cell capture. This method enables for continuous operation and, as well as, renders scaling and serial processing of cell samples—thereby being highly cost efficient.
As demonstrated in the study, DisCo exhibits accurate particle and cell positioning, and manages droplet sorting using combined multilayer and machine-vision microfluidics. These allow for processing of low-input single cell suspensions continuously at great capture efficiency of more than 70% at speeds that can reach 350 cells for an hour.
To showcase DisCo’s unique capabilities further, the scientists experimented it on the tiny chemosensory organs of the fruit fly Drosophila, and also on individual intestinal crypts and organoids. The latter are small tissues cultivated in culture dishes that closely resembles actual organs—a field that EPFL has been spearheading for several years.
The team employed DisCo to examine individual intestinal organoids at various developmental phases. By identifying several different organoid subtypes of which some was never been detected earlier, the technique presented a fascinating picture of heterogeneity in the organoids.
Our work demonstrates the unique ability of DisCo to provide high-resolution snapshots of cellular heterogeneity in small, individual tissues.”
Bart Deplancke, Professor, School of Life Sciences, École Polytechnique Fédérale de Lausanne
Source:
Journal reference:
Bues, J., et al. (2022) Deterministic scRNA-seq captures variation in intestinal crypt and organoid composition. Nature Methods. doi.org/10.1038/s41592-021-01391-1.