Mutations that cause muscle atrophy can be corrected using the CRISPR-Cas9 gene-editing tool. A group led by ECRC researcher Helena Escobar has now used mRNA to incorporate the tool into human muscle stem cells for the very first time, revealing a technique suitable for therapeutic strategies.
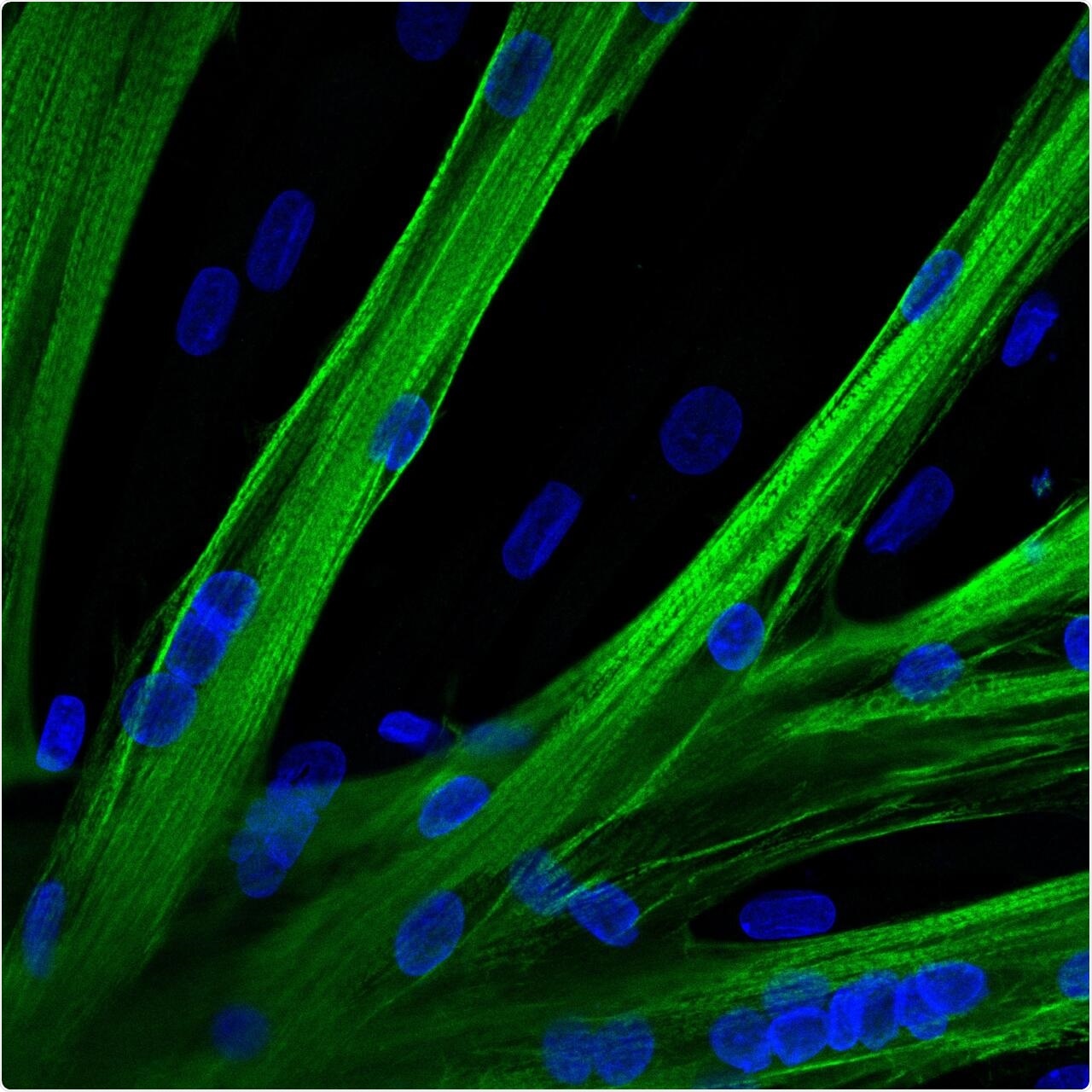
The researchers used mRNA to introduce the gene editor CRISPR-Cas9 into human muscle stem cells. These cells fused into multinucleated myotubes following mRNA-mediated CRISPR-Cas9 gene editing. A myosin heavy chain is seen in green and the nuclei in blue. Image Credit: Spuler Lab, MDC Berlin
It may only be a minor change in the genome, but this minor difference can be life-threatening: Almost all muscular dystrophies are triggered by a specific faulty gene. Regardless of how different the mutations in this sample of approximately 50 disorders are, they all result in a quite similar response.
Due to the genetic defect, changes occur in muscular structure and function so that sufferers experience progressive muscle atrophy.”
Simone Spuler, Professor and Head, Myology Lab, Experimental and Clinical Research Center
ECRC is a joint institution of the Berlin-based Max Delbrück Center for Molecular Medicine in the Helmholtz Association (MDC) and Charité – Universitätsmedizin Berlin. This condition is potentially fatal, particularly if the cardiac or respiratory muscles are disturbed.
The method has already proven successful in mice
Spuler and her colleagues want to dispute the fact that muscular dystrophies are currently incurable. Their most recent paper, published in the journal Molecular Therapy Nucleic Acids, lays the groundwork for a clinical study in which an ECRC-developed treatment will be tested on patients with hereditary muscle atrophy for the very first time.
Dr Helena Escobar, a postdoctoral researcher in Spuler’s lab and along with her co-last author of the current paper explains, “We have for several years been pursuing the idea of taking muscle stem cells from diseased patients, using CRISPR-Cas9 to correct the faulty genes, and then injecting the treated cells back into the muscles so that they can proliferate and form new muscle tissue.”
The researchers previously demonstrated that the approach worked in mice with muscle atrophy.
“Yet our method had a catch,” Escobar adds, interpreting “We introduced the genetic instructions for the gene editor into the stem cells using plasmids—which are circular, double-stranded DNA molecules derived from bacteria.”
However, plasmids may accidentally incorporate into the double-stranded genome of human cells, causing unwanted effects that are difficult to evaluate. “That made this method unsuitable for treating patients,” Escobar says.
Targeted correction of genetic defects
As a result, the researchers set out to find another solution. They discovered it in the type of messenger RNA (mRNA), a single-stranded RNA molecule that has gained significant attention as an important component of two Covid-19 vaccines.
In the vaccines, the mRNA molecules contain the genetic instructions for building the virus’s spike protein, which the pathogen uses to invade human cells.”
Christian Stadelmann, Doctoral Student, Spuler’s Lab, Max Delbrück Center for Molecular Medicine
Stadelmann is among the study’s co-lead authors, together with Silvia Di Francescantonio from the same group. “In our work we use mRNA molecules that contain the building instructions for the gene-editing tool.”
The scientists used electroporation, which partially makes cell membranes more susceptible to larger molecules, to have the mRNA into the stem cells. “With the help of mRNA containing the genetic information for a green fluorescent dye, we first demonstrated that the mRNA molecules entered almost all the stem cells,” Stadelmann explains.
The researchers then used a purposefully modified molecule on the surface of human muscle stem cells to demonstrate that the procedure could be used to rectify gene deformities in a targeted way.
A clinical trial is in the works
Ultimately, the researchers used a tool equivalent to the CRISPR-Cas9 gene editor, which does not split the DNA but only modifies it in one spot with extreme accuracy.
“This allows us to work with even greater precision, yet this tool is not suitable for every mutation that causes muscular dystrophy,” Stadelmann says.
In petri dish experiments, Stadelmann and his colleagues have now demonstrated that rectified muscle stem cells are just as skilled at fusing with one another and creating young muscle fibers as healthy cells.
“We are now planning to launch a first clinical trial with five to seven patients suffering from muscular dystrophy toward the end of the year,” Spuler adds.
Spuler also claims that the Paul-Ehrlich-Institut (PEI), Federal Institute for Vaccines and Biomedicines, which is in charge of clinical trial approval, has been supportive of the idea in an advisory meeting.
Of course, miracles cannot be expected, says Spuler, adding: “Sufferers who are in wheelchairs won’t just get up and start walking after the therapy. But for many patients, it is already a big step forward when a small muscle that is important for grasping or swallowing functions better again. The idea of repairing larger muscles, such as those needed for standing and walking, is already under consideration.”
However, for this to become a genuine therapy, the molecular tools need to become so secure that they can be presented without hesitation—not only into isolated muscle stem cells but also effectively into the degenerated muscle.
Source:
Journal reference:
Stadelmann, C., et al. (2022) mRNA-mediated delivery of gene editing tools to human primary muscle stem cells. Molecular Therapy Nucleic Acids. doi.org/10.1016/j.omtn.2022.02.016.