Scientists at UT Southwestern have discovered a four-protein complex that seems to play a significant function in the formation of ribosomes, which serve as protein factories for cells, as well as a surprise role in neurodevelopmental diseases. These results could lead to novel strategies to manipulate ribosome production, which could have implications for a range of human health disorders.
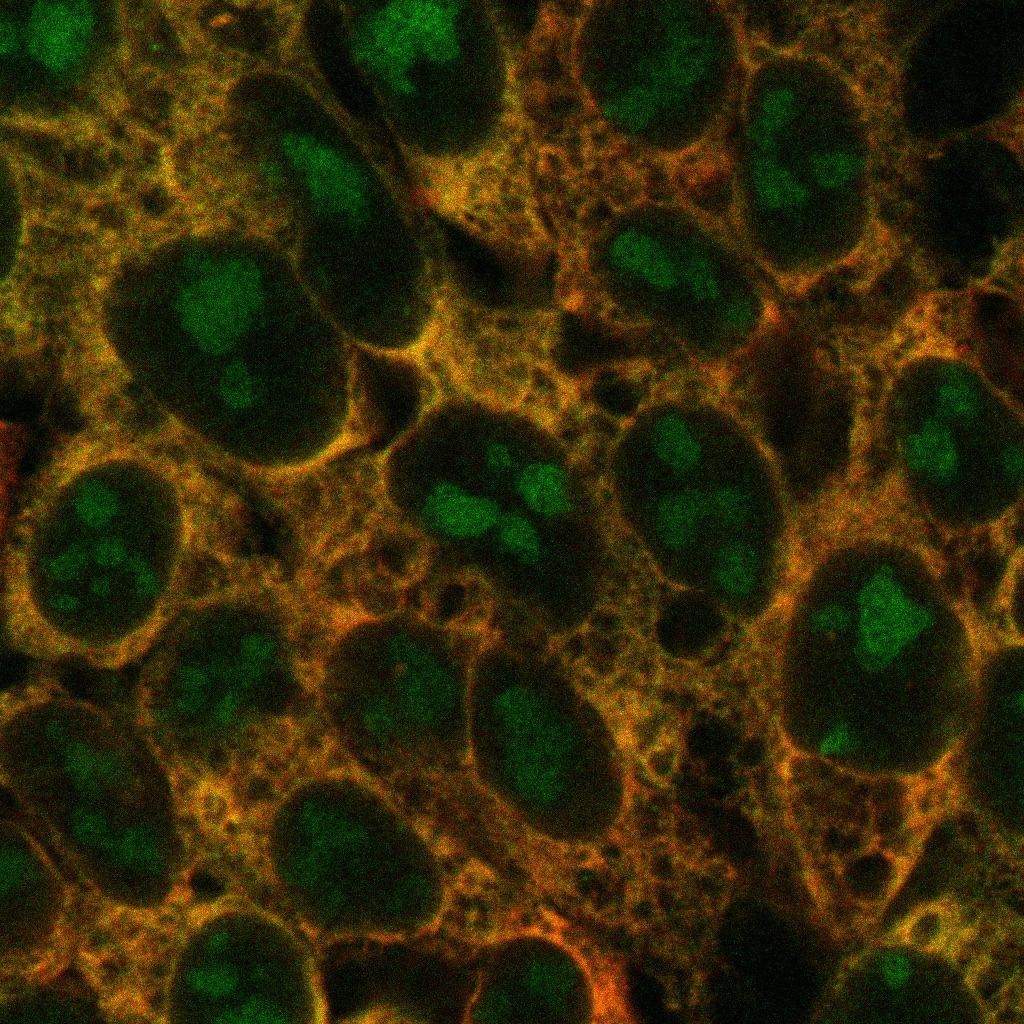 Human cells with new ribosomes (green), old ones (red), and overlap between the two (yellow). Labeling provides insights into the dynamics of ribosome production, which can vary between different cells. Image Credit: The University of Texas Southwestern Medical Center
Human cells with new ribosomes (green), old ones (red), and overlap between the two (yellow). Labeling provides insights into the dynamics of ribosome production, which can vary between different cells. Image Credit: The University of Texas Southwestern Medical Center
The study was published in the journal Cell Reports.
Ribosomes are fundamental for life, but we’ve had an incomplete understanding of how they’re put together and how the process of ribosome production is regulated. Our findings shed significant light on these questions.”
Michael Buszczak, PhD, Study Lead Author and Professor, Molecular Biology, University of Texas Southwestern Medical Center
Dr Buszczak was also a member of the Harold C. Simmons Comprehensive Cancer Center at UT Southwestern.
Ribosomes are found in variable numbers in every cell of just about every life on Earth, according to Dr Buszczak. Differences from these natural set points can have negative repercussions due to their important role as protein makers, the researcher warned. Cancer cells, for instance, accelerate ribosome production to promote protein output, which is required for unregulated cell division.
Furthermore, ribosomopathies, a group of rare disorders defined by aberrant ribosome production, can cause anemia, craniofacial deformities, and intellectual incapacity, among other symptoms.
Despite the fact that ribosomes are found in every species, most of what researchers understand about ribosome biogenesis comes from the yeast, a popular lab model. According to Dr Buszczak, the fundamentals of this mechanism are the same for human ribosome biogenesis, but the details are not. As a result, the characteristics that distinguish human ribosome production have remained a mystery.
Dr Buszczak, Chunyang Ni, a graduate student in the Buszczak lab, and their team members, including Jun Wu, PhD, Assistant Professor of Molecular Biology at UTSW, began by creating a methodology that caused old ribosomes to glow red and newly produced ribosomes to glow green to understand more about this process. The researchers tested this method on a variety of human cell types, finding that each has a different rate of ribosome manufacturing.
Individual genes were inactivated using the gene-editing technique CRISPR to determine those that might be important in ribosome biosynthesis. CINP, SPATA5L1, C1orf109, and SPATA5 were discovered throughout their investigation. Further research revealed that these genes form a complex that removes a placeholder protein from ribosomes when the assembly process is nearly complete, enabling another protein to fill that gap for ribosome maturation.
The role of SPATA5 in cells was unclear previously; however, mutations in this gene have been linked to neurodevelopmental diseases such as microcephaly, hearing problems, epilepsy, and cognitive impairment.
When the scientists injected two of these mutations into cells, leading them to produce a mutant SPATA5 protein, the cells were unable to produce the typical amount of functional ribosomes, implying that these neurodevelopmental diseases may be caused by ribosomes issues.
Dr Buszczak and his colleagues intend to investigate why the central nervous system seems to be more vulnerable to ribosomal disturbances than other cell types. He went on to say that these results could result in new treatments for cancer, ribosomopathies, and other diseases caused by excessive or insufficient protein production.
Source:
Journal reference:
Ni, C., et al. (2022) Labeling of heterochronic ribosomes reveals C1ORF109 and SPATA5 control a late step in human ribosome assembly. Cell Reports. doi.org/10.1016/j.celrep.2022.110597.