Researchers have long speculated an association between disorders in mitochondria, tiny organelles inside the cell, and the aging process and age-related illnesses, like Alzheimer’s disease.
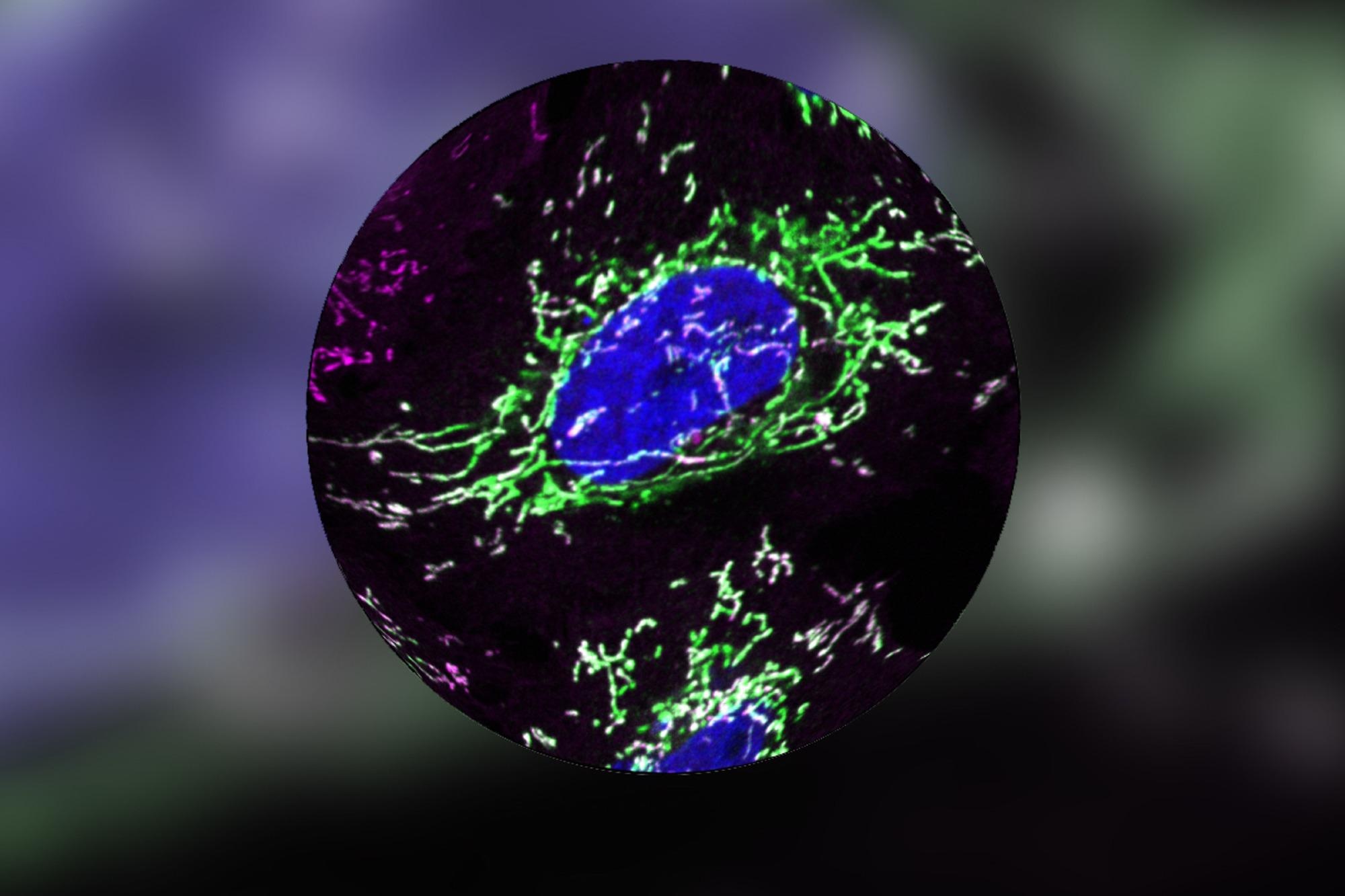 DELE1 (green) continuously migrates into mitochondria (magenta). It is poised to alert the nucleus (blue). Image Credit: E. Fessler, Jae Lab
DELE1 (green) continuously migrates into mitochondria (magenta). It is poised to alert the nucleus (blue). Image Credit: E. Fessler, Jae Lab
Many such illnesses cannot be cured—partly because we don’t yet understand fundamental mechanisms.”
Lucas Jae, Professor, Gene Center Munich, Ludwig-Maximilians-Universität München
Often, many forms of stress are responsible for activating mitochondrial dysfunction–this much is well-known. Stress might come either from the cell or originate in the mitochondrion itself, such as via reactive oxygen species, which take place during cellular respiration. Even though they have their genome, mitochondria lack the potential of responding individually to stress.
“This means that disturbances must be reported to the rest of the cell,” explained Dr Evelyn Fessler from the Gene Center Munich.
In the study published in the Nature Communications journal, Fessler and Jae, jointly with Luisa Krumwiede, explain a mechanism whereby a unique protein in humans, called DELE1, helps detect several types of stress while being imported into mitochondria and reports them to the cell. This can result in different responses, like induced cell death or repairs.
Known molecule, unknown mechanism
Two years back, a research group of Jae examined the question of how mitochondrial stress is reported to the cell. A new signaling pathway was identified by the scientists consisting of the proteins DELE1, HRI, and OMA1, which looks after these tasks.
So we knew which factors recognize mitochondrial stress, but we didn’t understand key aspects. How does the DELE1 signal travel from the mitochondrion into the cytoplasm of the cell? And how can DELE1, as an individual protein, detect the many different types of stress?”
Lucas Jae, Professor, Gene Center Munich, Ludwig-Maximilians-Universität München
Currently, scientists have found answers to their questions. DELE1 is constantly imported into the mitochondria and then processed by proteases. Furthermore, deep inside the mitochondria, DELE1 is rapidly degraded. As such, there is the existence of molecules that constantly passes via the inner and outer membranes of mitochondria to be imported.
Mitochondrial stress is responsible for the failure of this importing process. New DELE1 molecules have been arrested on their way into the mitochondria and, also based on the source of the disturbance, are either cut by OMA1 or tend to remain uncleaved outside the organelles. Anyway, the portion of the DELE1 protein that exhibits the signaling effect has been disclosed in the cytosol.
All the different types of stress lead to one of the sub-steps involved in the importing and processing of DELE1 coming to a halt.”
Lucas Jae, Professor, Gene Center Munich, Ludwig-Maximilians-Universität München
This is how the detection of mitochondrial stress has been done.
Also, DELE1 identifies dysfunctions in the mitochondrial enzymes named MPP and PITRM1. In neurodegenerative diseases, such enzymes are mutated.
Jae stated, “Specifically in connection with such defects, we have observed that it’s important for cellular survival for DELE1 to detect the problem and inform the cell.”
What takes place next? “Now that we understand the mechanism, we can investigate many different scenarios,” reported Fessler.
The scientists are willing to find out how the decision has been made as to if a cell enters a repair phase as a result of a stress response or goes into programmed cell death because otherwise, it would pose a threat.
Also, they hope to be able to regulate the signaling pathway such that it aids cellular survival in times of mitochondrial stress: a possible method for treating neurodegenerative diseases.
Source:
Journal reference:
Fessler, E., et al. (2022) DELE1 tracks perturbed protein import and processing in human mitochondria. Nature Communications. doi.org/10.1038/s41467-022-29479-y