Reviewed by Danielle Ellis, B.Sc.Apr 18 2022
Researchers aim to learn more about circadian rhythms, which are the 24-hour internal clock cycles of sleeping and waking that happens in all species, including humans, plants, fungi, and bacteria. A research group has studied the complicated workings of cyanobacteria, and they now have a better understanding of what motivates its circadian clock.
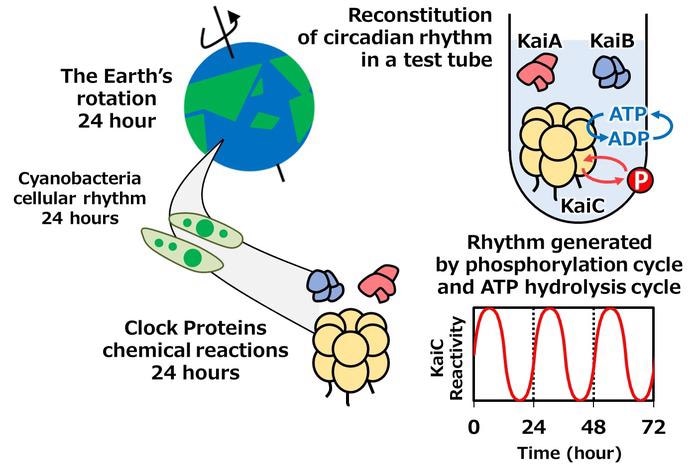 Circadian rhythms of the phosphorylation cycle (red circle with “P” indicating the phosphor transfer) and the ATP hydrolysis cycle (blue circle with “ATP” and “ADP” indicating the conversion of Adenosine-TriPhosphate into Adenosine-DiPhosphate) can be observed in a test tube. Image Credit: National Institutes of Natural Sciences
Circadian rhythms of the phosphorylation cycle (red circle with “P” indicating the phosphor transfer) and the ATP hydrolysis cycle (blue circle with “ATP” and “ADP” indicating the conversion of Adenosine-TriPhosphate into Adenosine-DiPhosphate) can be observed in a test tube. Image Credit: National Institutes of Natural Sciences
The team, led by academics from the National Institutes of Natural Sciences’ Institute for Molecular Science in Okazaki, Japan, reported their findings in Science Advances on April 15th, 2022.
KaiC, a clock protein that controls the circadian rhythm of cyanobacteria, a type of bacteria that exists in all sorts of water and is commonly found in blue-green algae, was the focus of the team’s investigation.
Proteins make up biological clocks in living things. The cyanobacterial circadian clock has the fewest components of any circadian clock, but it is nonetheless an extremely complicated system that can give scientists insight into how all circadian clocks function.
Image Credit: LeStudio/Shutterstock.com
Cyanobacteria are blueish microorganisms that can be found in a variety of settings, including salt and fresh waters, soils, and rocks. The researchers looked into the structural basis for allostery, as well as the complicated variations in shape and function of the KaiC protein in cyanobacteria. The cyanobacterial circadian clock is driven by allostery.
By testing thousands of crystallization conditions, the researchers were able to determine the atomic structures of the KaiC clock protein. They were able to cover the entire phosphorylation cycle, or the process of transferring phosphate to a protein, thanks to their in-depth analysis of the atomic structures.
Phosphorylation works in tandem with ATP hydrolysis, which is the energy-intensive step that determines the clock speed. The phosphorylation-ATP hydrolysis system acts as a cell activity controller.
They crystallized the KaiC protein in eight different states to better comprehend the idea of allostery, enabling them to see how the phosphorylation cycle and the ATP hydrolysis cycle function together like two gears.
Researchers have previously investigated the KaiC protein’s phosphorus cycle in vivo, in vitro, and in silico. Allostery governs the phosphorus cycle in KaiC, but little is known about it.
The team was able to detect a link between the phosphorus cycle and the ATPase hydrolysis cycle by analyzing the KaiC in eight different states. The cyanobacterial circadian clock is driven by the interaction of the two gears.
Because proteins are composed of a vast number of atoms, it is not easy to understand the mechanisms of their complicated but ordered functions. We need to trace the structural changes of proteins patiently.”
Yoshihiko Furuike, Assistant Professor, Institute for Molecular Science, National Institutes of Natural Sciences
The KaiC protein regulates the assembly states of other clock-related proteins by rhythmically activating and inactivating reaction cycles on its own. Therefore, as they consider their next approaches, the researchers may turn to structural biology to uncover the atomic causes of gear rotation acceleration and deceleration.
Our goal is to see all cyanobacterial clock proteins during the oscillation at an atomic level and to describe the moment that the ordered rhythm arises from chaotic atomic dynamics.”
Yoshihiko Furuike, Assistant Professor, Institute for Molecular Science, National Institutes of Natural Sciences
Their findings could be used as a research tool, allowing scientists to gain a better understanding of the systems that govern the circadian clock cycle. The research team anticipates that their findings will have broader implications in the future.
Clock proteins exist in mammals, insects, plants, and microorganisms, each with its patterns and configurations. “However, the logic behind the relationship between KaiC dynamics and clock functions can be applied to other studies on various organisms,” Furuike said.
Source:
Journal reference:
Furuike, Y., et al. (2022) Elucidation of master allostery essential for circadian clock oscillation in cyanobacteria. Science Advances. doi.org/10.1126/sciadv.abm8990.