Humans live in a world created and managed by RNA—the genetic molecule DNA’s equally important sibling. Evolutionary scientists believe that RNA existed and was self-replicated before DNA was discovered.
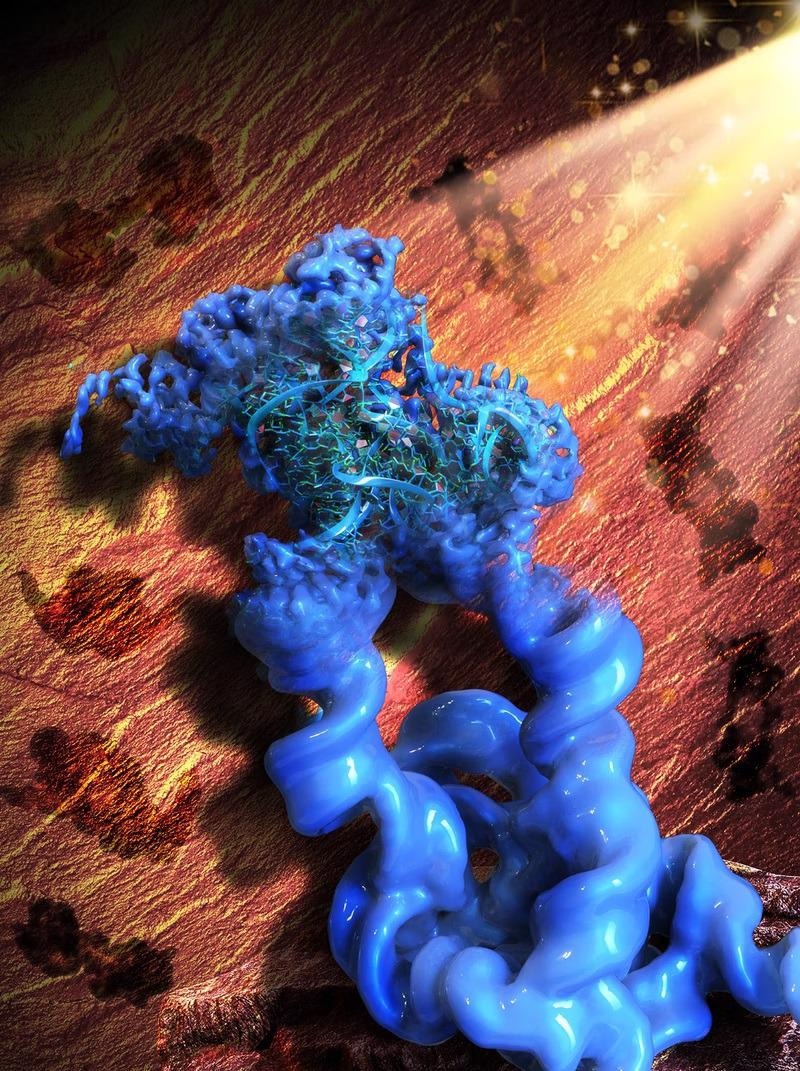 This illustration is inspired by the Paleolithic rock painting in the Lascaux cave, signifying the acronym of our method, ROCK. Figuratively, the patterns of the rock art in the background (brown) are the 2D projections of the engineered dimeric construct of the Tetrahymena group I intron, while the main object in the front (blue) is the reconstructed 3D cryo-EM map of the dimer, with one monomer in focus and refined to the high resolution that allowed the collaborators to build an atomic model of the RNA. Image Credit: Wyss Institute at Harvard University.
This illustration is inspired by the Paleolithic rock painting in the Lascaux cave, signifying the acronym of our method, ROCK. Figuratively, the patterns of the rock art in the background (brown) are the 2D projections of the engineered dimeric construct of the Tetrahymena group I intron, while the main object in the front (blue) is the reconstructed 3D cryo-EM map of the dimer, with one monomer in focus and refined to the high resolution that allowed the collaborators to build an atomic model of the RNA. Image Credit: Wyss Institute at Harvard University.
Moving forward to modern-day people, scientists have discovered that just around 3% of the human genome is translated into messenger RNA (mRNA) molecules, which are then translated into proteins. In contrast, 82% of it is translated into RNA molecules, which have a variety of additional activities, many of which are yet unknown.
The 3D structure of each RNA molecule must be decoded at the level of its component atoms and molecular bonds to comprehend what it performs. DNA and protein molecules have been frequently analyzed by converting them into regularly packed crystals that may be inspected with an X-ray beam (X-ray crystallography) or radio waves (nuclear magnetic resonance).
These methods, however, cannot be used as effectively on RNA molecules due to their molecular composition and structural flexibility, which prevents them from forming crystals easily.
A novel method for the structural examination of RNA molecules has been published by a research team led by Wyss Core Faculty member Peng Yin, PhD at Harvard University’s Wyss Institute for Biologically Inspired Engineering, and Maofu Liao, PhD at Harvard Medical School (HMS).
ROCK is an RNA nanotechnological technique that allows it to combine many identical RNA molecules into a highly ordered structure, reducing the flexibility of individual RNA molecules and multiplying their molecular weight.
Using well-known model RNAs of various lengths and functions as benchmarks, the researchers demonstrated that their method allows structural characterization of the included RNA subunits using the cryo-electron microscopy technique (cryo-EM). Nature Methods has reported on their progress.
ROCK is breaking the current limits of RNA structural investigations and enables 3D structures of RNA molecules to be unlocked that are difficult or impossible to access with existing methods, and at near-atomic resolution. We expect this advance to invigorate many areas of fundamental research and drug development, including the burgeoning field of RNA therapeutics.”
Peng Yin, PhD, Study Author and Wyss Core Faculty member, Wyss Institute for Biologically Inspired Engineering at Harvard University
Yin is also a Professor in the Department of Systems Biology at HMS and a leader of the Wyss Institute’s Molecular Robotics Initiative.
Gaining control over RNA
DNA bricks and DNA origami are two technologies developed by Yin’s team at the Wyss Institute that allow DNA and RNA molecules to self-assemble into massive structures based on distinct principles and needs. Researchers theorized that by precisely binding RNA molecules together, similar techniques may be utilized to assemble naturally existing RNA molecules into highly structured circular complexes in which their flexibility to flex and move is severely limited.
Many RNAs fold in intricate but predictable patterns, with tiny segments base-pairing. A stable “core” and “stem-loops” spilling out onto the periphery are frequently the consequence.
In our approach we install ‘kissing loops’ that link different peripheral stem-loops belonging to two copies of an identical RNA in a way that allows an overall stabilized ring to be formed, containing multiple copies of the RNA of interest. We speculated that these higher-order rings could be analyzed with high resolution by cryo-EM, which had been applied to RNA molecules with first success.”
Di Liu, PhD, Study First Author and Postdoctoral Fellow, Wyss Institute for Biologically Inspired Engineering at Harvard University
Picturing stabilized RNA
Multiple single particles are flash-frozen at cryogenic temperatures to inhibit further movement, then observed using an electron microscope and computational algorithms that compare various elements of a particle’s 2D surface projections and rebuild its 3D architecture. Peng and Liu collaborated on the work alongside Liao and François Thélot, PhD, a former graduate student of Liao’s.
Liao and research colleagues have made significant contributions to the fast-developing cryo-EM field, as well as the experimental and computational characterization of single particles generated by specific proteins.
Cryo-EM has great advantages over traditional methods in seeing high-resolution details of biological molecules including proteins, DNAs and RNAs, but the small size and moving tendency of most RNAs prevent successful determination of RNA structures. Our novel method of assembling RNA multimers solves these two problems at the same time, by increasing the size of RNA and reducing its movement.”
Maofu Liao, PhD, Associate Professor, Cell Biology, Blavatnik Institute, Harvard Medical School
Liao adds, “Our approach has opened the door to rapid structure determination of many RNAs by cryo-EM.” The scientists named their method after combining RNA nanotechnology with cryo-EM technologies “RNA oligomerization-enabled cryo-EM via installing kissing loops” (ROCK).
The scientists focused on a big intron RNA from Tetrahymena, a single-celled organism, and a short intron RNA from Azoarcus, a nitrogen-fixing bacteria, as well as the so-called FMN riboswitch, to offer proof-of-principle for ROCK. Non-coding RNA sequences called intron RNAs are found throughout the sequences of freshly transcribed RNAs and must be “spliced” removed before the mature RNA can be produced.
The FMN riboswitch is present in bacterial RNAs that are involved in the manufacture of vitamin B2 flavin metabolites. It alters its 3D shape and inhibits the production of its mother RNA when it binds one of them, flavin mononucleotide (FMN).
“The assembly of the Tetrahymena group I intron into a ring-like structure made the samples more homogenous, and enabled the use of computational tools leveraging the symmetry of the assembled structure. While our dataset is relatively modest in size, ROCK’s innate advantages allowed us to resolve the structure at an unprecedented resolution,” stated Thélot.
Thélot also added, “The RNA’s core is resolved at 2.85 Å [one Ångström is one ten-billions (US) of a meter and the preferred metric used by structural biologists], revealing detailed features of the nucleotide bases and sugar backbone. I don’t think we could have gotten there without ROCK–or at least not without considerably more resources.”
Cryo-EM may also capture molecules in various states if they change their 3D confirmation as part of their function, for example. Using ROCK on the Azoarcus intron RNA and the FMN riboswitch, the researchers were able to detect the many conformations that the Azoarcus intron changes throughout its self-splicing process, as well as the relative conformational stiffness of the FMN riboswitch’s ligand-binding site.
Donald Ingber, MD, PhD, stated, “This study by Peng Yin and his collaborators elegantly shows how RNA nanotechnology can work as an accelerator to advance other disciplines. Being able to visualize and understand the structures of many naturally occurring RNA molecules could have tremendous impact on our understanding of many biological and pathological processes across different cell types, tissues, and organisms, and even enable new drug development approaches.”
Ingber is also Wyss Founding Director and Judah Folkman Professor of Vascular Biology at the Harvard Medical School and Hansjörg Wyss Professor of Bioinspired Engineering at the Harvard John A. Paulson School of Engineering and Applied Sciences, as well as a Vascular Biology Program at Boston Children’s Hospital.
Source:
Journal reference:
Liu, D., et al. (2022) Sub-3-Å cryo-EM structure of RNA enabled by engineered homomeric self-assembly. Nature Methods. doi.org/10.1038/s41592-022-01455-w.