Clustered regularly interspaced short palindromic repeats (CRISPR) and their accompanying protein, CRISPR-associated protein 9 (Cas9), several years ago, made international news as a game-changing genome editing system.
 Fifth-generation polyrotaxane (PRX) carriers can effectively deliver CRISPR-Cas9 ribonucleoproteins (RNPs). Image Credit: Kumamoto University
Fifth-generation polyrotaxane (PRX) carriers can effectively deliver CRISPR-Cas9 ribonucleoproteins (RNPs). Image Credit: Kumamoto University
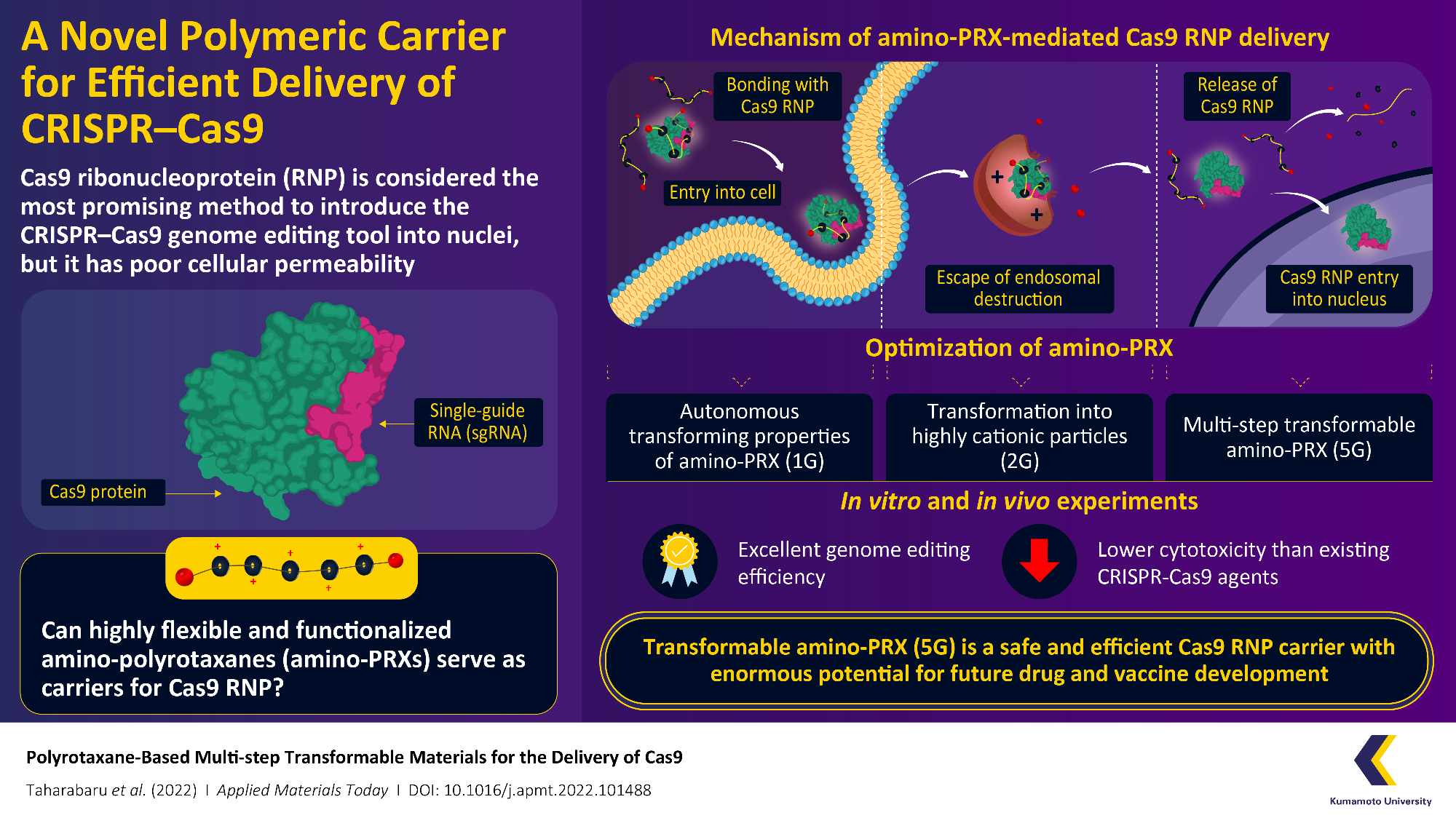
The system, which consists of Cas9 and a strand of genetic material defined as a single-guide RNA (sgRNA), can specifically target regions of DNA and act as “molecular scissors” to make accurate edits. The immediate delivery of Cas9–sgRNA complexes, i.e. Cas9 ribonucleoproteins (RNPs), into the cell nucleus is thought to be the best and safest method of genome editing.
The Cas9 RNP, on the other hand, has low cellular permeability and thus demands a carrier molecule to transmit it well beyond the first hurdle of the cell membrane before reaching the nucleus. These carriers must connect to Cas9 RNP, transport it into the cell, prevent degradation by intracellular organelles known as “endosomes,” and eventually release it without causing structural changes.
A research group from Kumamoto University established a transformable polyrotaxane (PRX) carrier that can enable genome editing using Cas9RNP with good precision and functionality. The study was published in a recent paper in June 2022 in Volume 27 of Applied Materials Today.
While there have been some PRX-based drug carriers for nucleic acids and proteins reported before, this is the first report on PRX-based Cas9 RNP carrier. Moreover, our findings describe how to precisely control intracellular dynamics across multiple steps. This will prove invaluable for future research in this direction.”
Keiichi Motoyama, Study Corresponding Author and Professor, Kumamoto University
The research group concentrated on PRX with amine groups, i.e. amino-PRX, for their new carrier and went through multiple shots of design and optimization before obtaining their finished version. The first generation (1G) of their carrier molecules, for instance, used amino-autonomous PRX’s transforming characteristics to effectively complex it with Cas9 RNP and facilitate its transmission past the cell membrane.
The second-generation (2G) concentrated on endosome escape. This was accomplished by converting the amino groups in amino-PRX into strongly cationic (positively charged) particles within the endosome, causing the endosome to rupture and Cas9 RNP–amino-PRX to evacuate.
The following generations discussed issues related to Cas9 release after the complex had fled the endosome. Eventually, researchers created the fifth generation (5G) multi-step transformable amino-PRX carrier, which was capable of efficiently and precisely delivering Cas9 RNP into the cell nucleus. The researchers then conducted in vitro and in vivo experiments to verify the system’s cytotoxicity as well as its genome editing performance.
Our delivery system has a low cytotoxicity and its genome editing activity is equal to the current most efficient system on the market.”
Taishi Higashi, Study Corresponding Author and Associate Professor, Kumamoto University
“Moreover, our multiple attempts at optimizing the delivery system across generations offer important information on the types and positions of various biodegradable groups and amino groups that can be used in such a system to further customize and adapt their properties,” adds Higashi.
The 5G amino-PRX carrier’s independent action, multi-step transformable characteristics, and low cytotoxicity make it a highly promising candidate for the secure and convenient delivery of Cas9 RNP. These results could also be used to deliver a variety of molecules like enzymes, antibodies, and small interfering RNA (siRNA), producing this novel carrier a significant accomplishment in the profession of drug and vaccine advancement.
Source:
Journal reference:
Taharabaru, T., et al. (2022) Polyrotaxane-based multi-step transformable materials for the delivery of Cas9 ribonucleoprotein. Applied Materials Today. doi.org/10.1016/j.apmt.2022.101488.