Reviewed by Danielle Ellis, B.Sc.May 30 2022
The Chinese Academy of Sciences’ Suzhou Institute of Biomedical Engineering and Technology recently reported that the DMDRMR/miR-378a-5p/ DAB2IP axis enhances angiogenesis and sunitinib resistance, suggesting that it could be used as a diagnostic, prognostic, and therapeutic goal for patients with clear cell renal cell carcinoma (ccRCC).
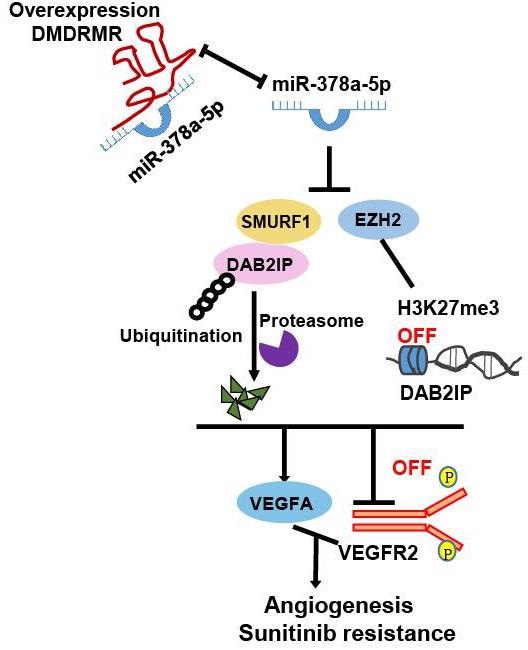 Proposed model for the DMDRMR/miR-378a-5p/DAB2IP axis promoting the angiogenesis and sunitinib resistance of ccRCC. Image Credit: SIBET
Proposed model for the DMDRMR/miR-378a-5p/DAB2IP axis promoting the angiogenesis and sunitinib resistance of ccRCC. Image Credit: SIBET
These findings complement and expand on their past work.
ccRCCs are a common kind of RCC that are known for being extremely angiogenic and vascularized. Certain essential genes, such as the Hippel-Lindau tumor suppressor gene, are frequently altered in RCC, resulting in aberrant activation of downstream proteins and signaling pathways and neovascularization, encouraging tumorigenesis, progression, and metastasis.
As a result, for patients with severe RCC, targeted angiogenesis inhibitors like sunitinib and sorafenib have been employed as first-line treatments. The majority of patients, however, gain medication resistance to the angiogenesis therapy. As a result, a better knowledge of tumor angiogenesis in ccRCC is essential.
The greatest vascular growth factor in promoting tumor angiogenesis is vascular endothelial cell growth factor A (VEGFA), a member of the VEGF family. VEGFA, which stimulates endothelial cell differentiation, proliferation, migration, and angiogenesis by attaching to its major receptor VEGFR2, is highly expressed in tumor cells. As a result, the VEGFA/VEGFR2 signaling pathways are important in tumor angiogenesis.
Last year, the scientists found that a long non-coding RNA known as DNA methylation-deregulated and RNA m6A reader-cooperating lncRNA (DMDRMR) acts as a cofactor for insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3), allowing it to stabilize target genes in an m6A-dependent manner, and therefore plays important oncogenic roles in ccRCC.
They also discovered that DMDRMR enhances VEGFA expression and affects angiogenesis-related biological processes, but that these actions are autonomous of its binding protein IGF2BP3. As a result, a modern research question has arisen: does DMDRMR stimulate neovascularization in ccRCC, and if so, how?
The scientists concluded that DMDRMR acts as a miR-378a-5p sponge, increasing the expression of the enhancer of zeste homolog 2 (EZH2) and smad ubiquitination regulatory factor 1 (SMURF1), boosting EZH2-mediated transcriptional repression of DOC-2/DAB2 interactive protein (DAB2IP) and SMURF1-mediated degradation of DAB2IP.
As a result, in ccRCC, this axis triggers the VEGFA/VEGFR2 signaling pathway, promoting angiogenesis and tumor cell resistance to sunitinib.
They also discovered that the DMDRMR/miR-378a-5p/IGF2BP3 axis has high expression and a good correlation with ccRCC patients and that the combination of overexpressed DMDRMR and negatively regulated DAB2IP anticipated the poorest overall survival of ccRCC patients, implying that the axis could be a new target for ccRCC patients’ mixture diagnosis/therapy.
These findings could have many clinical implications for developing tailored therapy for patients with ccRCC in the coming years.
Source:
Journal reference:
Zhu, Y., et al. (2022) DMDRMR promotes angiogenesis via antagonizing DAB2IP in clear cell renal cell carcinoma. Cell Death & Disease. doi.org/10.1038/s41419-022-04898-3.