Scientists from Kumamoto University, the National Institute of Genetics (Japan), and the University of Michigan (USA) used next-generation sequencing to analyze human endogenous retrovirus (HERV) integration sites and found that these primitive retroviruses can retrotranspose (DNA sequence insertion with RNA mediation) into iPS cells.
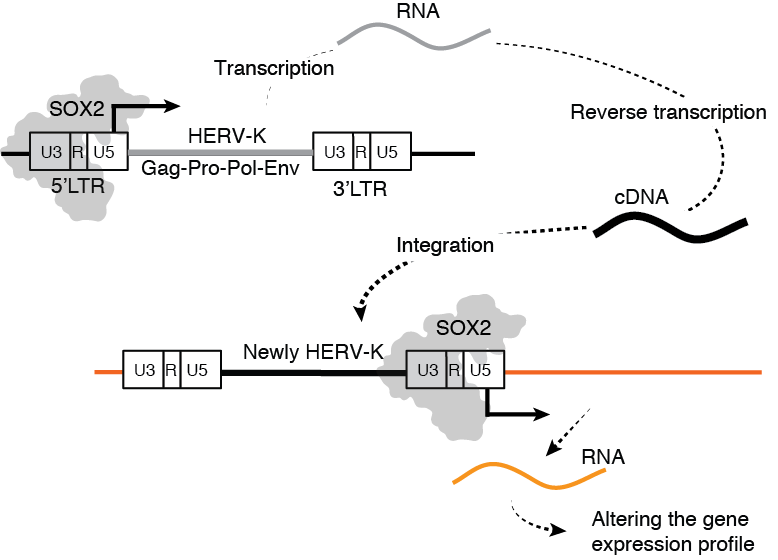 HERV-K is expressed by SOX2 and moves on the genome through reverse transcription and integration. Image Credit: Kumamoto University
HERV-K is expressed by SOX2 and moves on the genome through reverse transcription and integration. Image Credit: Kumamoto University
The researchers feel that their observation highlights a potential HERV risk when employing iPS cells in regenerative medicine.
The study of ancient retroviruses buried in human DNA necessitates an understanding of the history of coexistence with viral dangers. Researchers know that HERVs take up around 8% of the human genome and acquire mutations and deletions over extended periods. Early embryos express HERVs, which serve a variety of physiological roles in human evolution.
For instance, HERV-W and HERV-FRD Env proteins are necessary for placental development and HERV-K is hypothesized to defend host cells from external retrovirus infection. Uncontrollable HERV-K expression is also suspected to be linked to a variety of diseases, particularly cancer and neurological disorders, but the intricacies of this link in humans are unknown.
Since no replication-competent HERVs have been detected in the genome, they are assumed to be from an extinct (fossil) virus. HERV-K is expressed in SOX2-expressing cells, like those in early embryos, cancer stem cells, and iPS cells, according to a study team from Japan and the United States. They also discovered that in the lack of Env, the viral envelope glycoprotein, some HERV-K is freshly incorporated into the host DNA.
Since reverse transcriptase, integrase, and protease were required for this insertion, the scientists speculate that the HERV-K buried in the human genome is not a fossil virus, but rather travels on the genome through the creation of proviral DNA reverse transcription.
When the scientists evaluated the HERV-K integration sites in iPS and fibroblast cells from the same donor, they discovered that iPS cells had new HERV-K integration sites. The new integration site, on the other hand, was rarely retained and eventually vanished during long-term culturing. Since HERV-K is expected to be spontaneously incorporated into the genome, HERV-K retrotransposed cells may survive primarily due to their integration site.
By modifying the gene expression profile, HERV-K migration on the genome may induce cancer and neurological illnesses. Although the researchers feel that the danger of HERV-K transposition in iPS cells is low, they recommend that tracking HERV-K integration sites be carefully examined to increase the efficacy of regenerative therapy employing iPS cells.
Source:
Journal reference:
Monde, K., et al. (2022) Movements of Ancient Human Endogenous Retroviruses Detected in SOX2-Expressing Cells. Virology. doi.org/10.1128/jvi.00356-22.