Proteins are made in cells in the same way as they are made in factories. However, making too much at the wrong moment may lead to illnesses like cancer, so they use RNA interference (RNAi) to regulate production. RNAi is already being used to address painful kidney and liver diseases, with additional seven drugs in clinical studies as of July 2021.
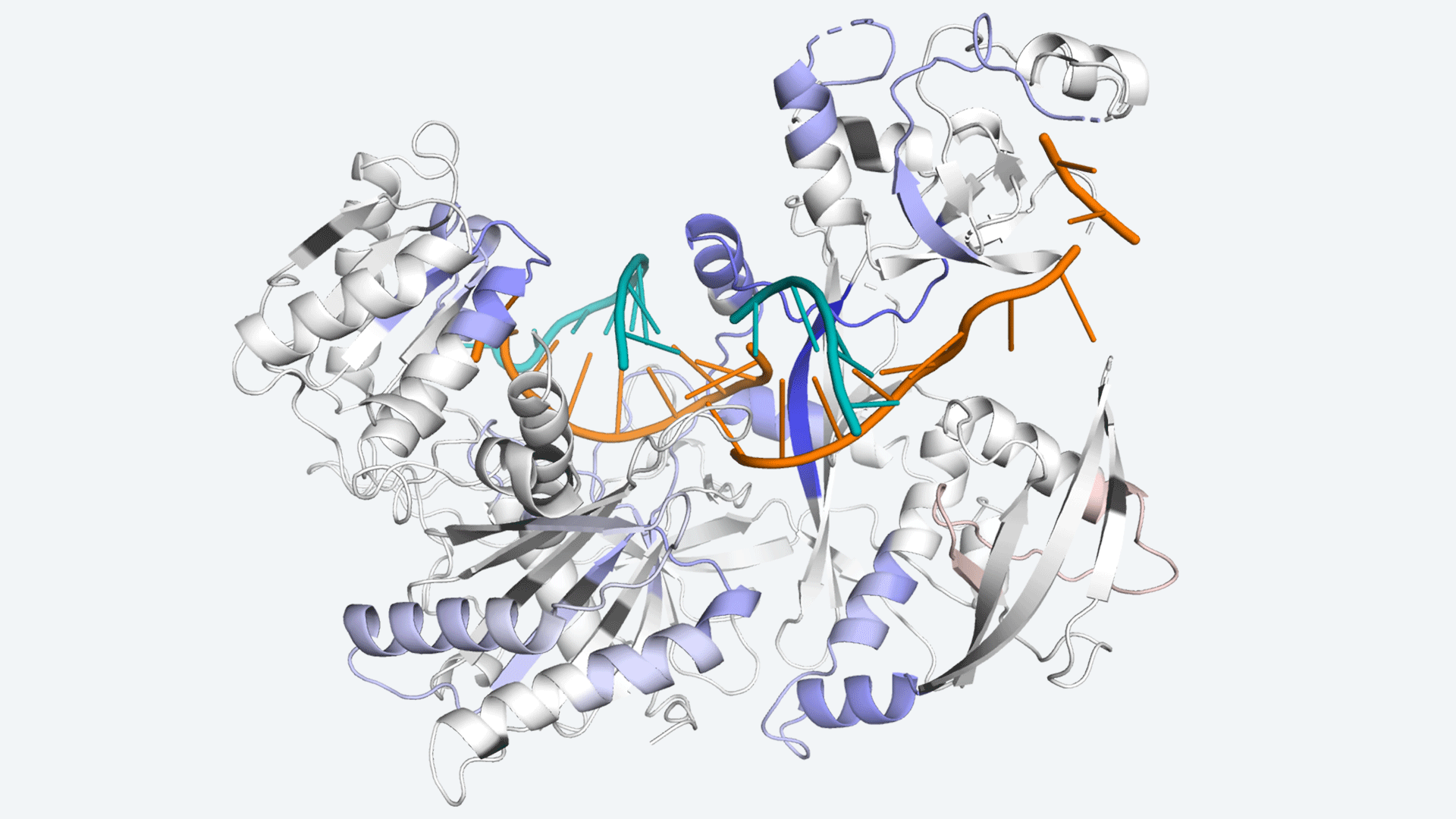 The protein Argonaute, which helps cells control protein production in a process called RNA interference. Image Credit: In conjunction with Scripps Research.
The protein Argonaute, which helps cells control protein production in a process called RNA interference. Image Credit: In conjunction with Scripps Research.
RNAi medicines have a lot of promise, and researchers at Cold Spring Harbor Laboratory (CSHL) are working hard to create a full picture of the process in order to enhance current therapies and develop better ones in the future.
Leemor Joshua-Tor, CSHL Professor, and HHMI Investigator and Brianna Bibel, a recent CSHL School of Biological Sciences graduate, are filling in some of the gaps. Researchers just found how Argonaute (Ago), an RNAi workhorse protein, makes use of limited resources to keep protein synthesis on track.
Because RNAi is such a fundamental and widely utilized technology, Joshua-Tor believes it is critical to fully comprehend how it operates. It also provides a form of a therapeutic safety net because it does not create lasting cell alterations and may be reversed.
For therapeutics, you’d kinda maybe not wanna mess around with the genome so much. In all these kinds of things, you wanna know exactly what’s happening, and if something isn’t working, then you know what to do and where to look. The more information you have, the better it is—you get a complete picture of what’s happening.”
Leemor Joshua-Tor, Professor, Cold Spring Harbor Laboratory and Investigator, Howard Hughes Medical Institute
Ago aids in the reduction of protein synthesis by locating, binding, and deleting molecules known as mRNA, which instruct cells to produce proteins. The amount of Ago in the body, however, is small as compared to the amount of mRNA it must target.
The protein is still capable of locating another after killing one, but it cannot proceed forward without assistance. Bibel revealed how cells employ phosphorylation to remove Ago’s hold on one mRNA target and let it travel to the next.
Our theory is that having phosphorylation promote release is a way that you could free up Argonaute because when the target gets released, the guide’s still there and it’s super-duper stable. So our thinking is that by phosphorylating it, you’re going to free it to go repress other targets—because it’s still totally capable of doing that work.”
Brianna Bibel, Graduate, School of Biological Sciences, Cold Spring Harbor Laboratory
Bibel expects that their discoveries will be useful as RNAi research develops.
A lot of great advances in science come from just doing basic research. And this is one of those basic research questions, trying to figure out how this is working.”
Brianna Bibel, Graduate, School of Biological Sciences, Cold Spring Harbor Laboratory
Source:
Journal reference:
Bibel, B., et al. (2022) Target binding triggers hierarchical phosphorylation of human Argonaute-2 to promote target release. eLife. doi.org/10.7554/eLife.76908.