The research, headed by Professor Michael Schrader of the University of Exeter, looked at peroxisome dynamics and discovered new ways for them to divide.
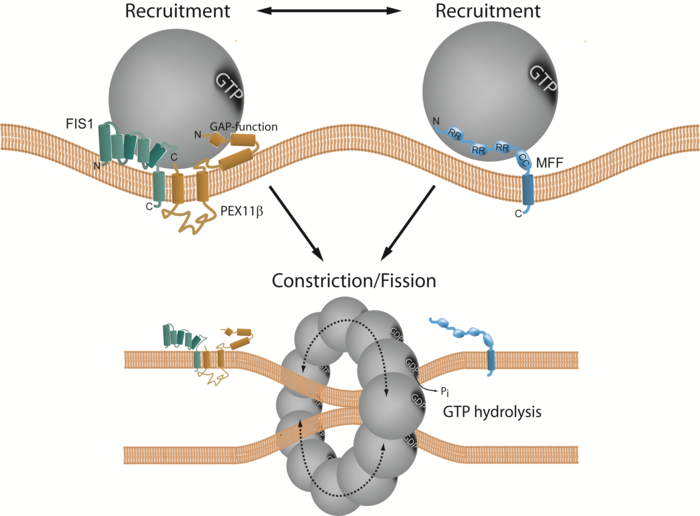 Working model of PEX11β-FIS1-dependent and MFF-dependent peroxisome division. The fission GTPase DRP1 is shown in grey. Image Credit: University of Exeter
Working model of PEX11β-FIS1-dependent and MFF-dependent peroxisome division. The fission GTPase DRP1 is shown in grey. Image Credit: University of Exeter
Organelles are a cell’s functional units. They have specialized tasks, and abnormalities in the enzymes that carry out those jobs might cause metabolic problems.
Organelles, on the other hand, are very dynamic, allowing them to reproduce by expanding and then dividing to adapt their number and functions to alter cellular needs.
Image Credit: Christoph Burgstedt/Shutterstock.com
Researchers have discovered a novel group of illnesses characterized by abnormalities in membrane dynamics and organelle division rather than metabolic function loss.
Organelle division problems cause developmental and neurological issues in patients.
Mutations in genes encoding organelle division machinery, such as Mitochondrial fission factor (MFF), a crucial component of the division mechanism of two organelles, mitochondria, and peroxisomes, are the cause of several illnesses.
MFF acts as an adapter protein, attracting Dynamin-related protein 1 (DRP1), a mechanochemical enzyme, to mitochondria and peroxisomes.
Membrane fission and organelle proliferation require this enzyme, which may constrict and divide membranes.
Peroxisomes (and mitochondria) in patient cells become extremely elongated when MFF function is lost.
As a result, it was hypothesized that MFF is the most important component in peroxisome division.
Professor Schrader leads an international, multi-disciplinary team of scientists that have discovered a new path for their division.
Peroxisomes play crucial protective roles in the cell and are essential for health—they play a role in cellular lipid metabolism and redox equilibrium, which connects them to energy regulation, cellular aging, and age-related diseases.
In this study, we show that the peroxisomal membrane protein PEX11β can divide the highly elongated, fission-incompetent peroxisomes in MFF-deficient cells when the PEX11β levels are increased.”
Michael Schrader, Professor, University of Exeter
“However, PEX11β does not have the ability to divide peroxisomes on its own. It requires FIS1 (Fission factor 1), another membrane adaptor for DRP1, whose exact role was previously unclear,” he said.
Interestingly, we observed that increased levels of MFF can also divide peroxisomes into PEX11β-deficient cells and restore a normal peroxisome morphology.”
Tina Schrader, Study First Author and Senior Research Technician, University Of Exeter
Patients with PEX11β dysfunction have a small stature, increasing hearing loss, and neurological problems.
We show that alternative pathways for peroxisome division exist; one using MFF and DRP1, and another using PEX11β, FIS1, and DRP1.”
Dr Ruth Carmichael, Study First Author, University Of Exeter
Professor Michael Schrader explained, “Our observations suggest that modulation of MFF or PEX11β protein levels may represent a therapeutic option to overcome the defects in peroxisome dynamics.”
“Pharmacological agents that up-regulate MFF or PEX11β may therefore be of therapeutic value to restore peroxisome dynamics in certain disease conditions. This might also be relevant to age-related conditions like dementia, deafness, and blindness, as peroxisomal dynamics are known to have important protective functions within sensory cells,” he added.
People with severe peroxisomal illnesses, often known as Zellweger Spectrum Disorders, frequently die as children or young adults, and the group collaborates with the Zellweger UK charity to promote awareness and support families and suffering.
Source:
Journal reference:
Schrader, T. A., et al. (2022) New research gives insights into how organelles divide into cells. Journal of Cell Science. doi.org/10.1242/jcs.259924.