Reviewed by Danielle Ellis, B.Sc.Jun 13 2022
The identification of pathogen-associated molecular patterns (PAMPs) by pattern-recognition receptors (PRRs) is the first step in initiating an immune response to viral infection. RIG-I, MDA5, and cGAS are the most important PRRs for detecting viral nucleic acids. Because polyubiquitination and deubiquitination are critical for their activation, it is crucial to understand how ubiquitinase and deubiquitinase govern RIG-I, MDA5, and cGAS.
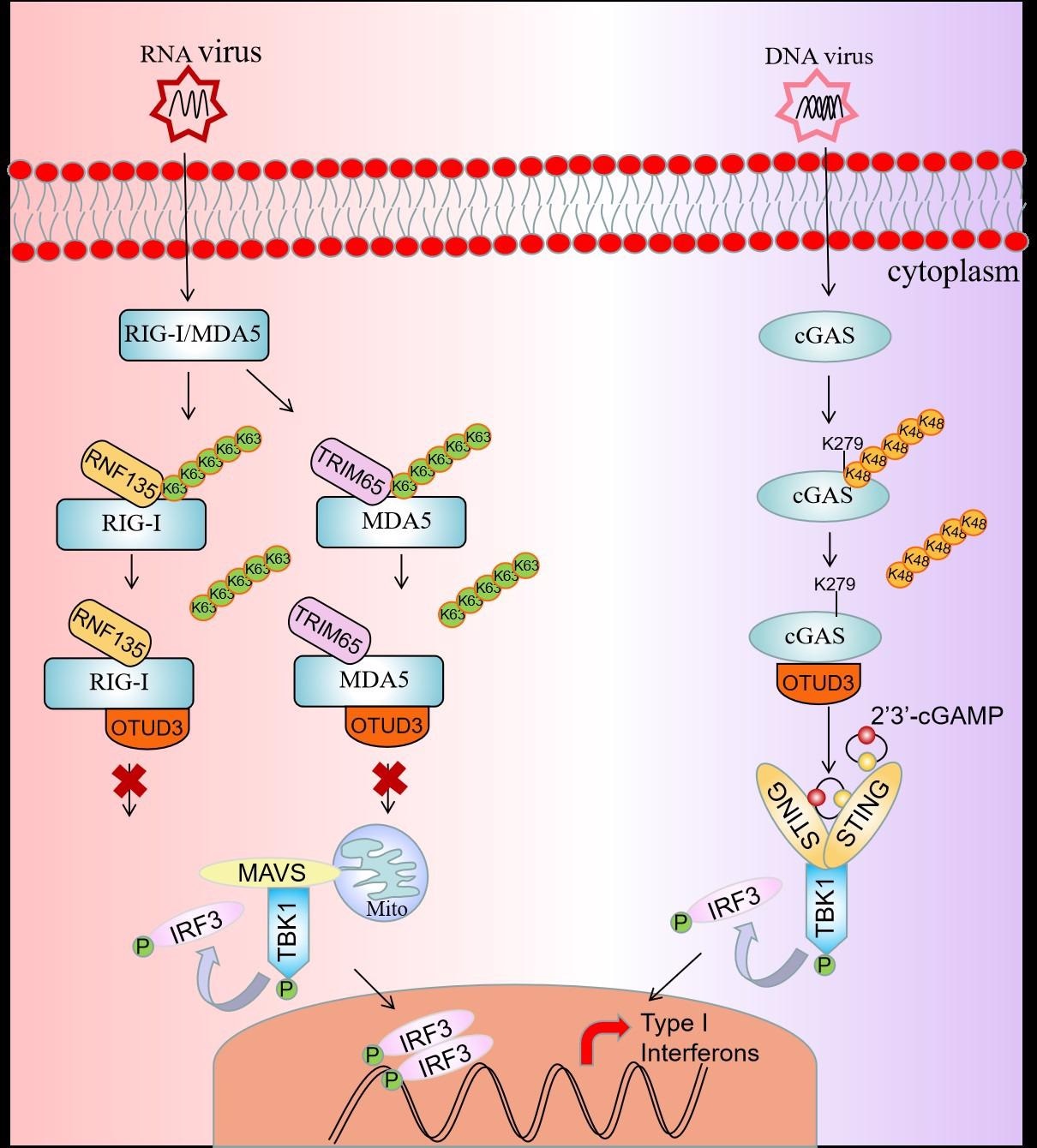 Graphical abstract of deubiquitinase OTUD3 in antiviral innate immunity. Image Credit: Institute of Hydrobiology.
Graphical abstract of deubiquitinase OTUD3 in antiviral innate immunity. Image Credit: Institute of Hydrobiology.
Prof. Wuhan Xiao of the Chinese Academy of Sciences’ Institute of Hydrobiology (IHB) has discovered that the deubiquitinase OTUD3 triggers a distinct immune response depending on whether it is reacting to an RNA or DNA virus. OTUD3 suppresses RNA virus-induced innate immunity in the first example but increases DNA virus-induced innate immunity in the latter. The research was published in the journal Cell Reports.
The researchers employed ubiquitination experiments to discover that overexpression of OTUD3 reduced K63-linked polyubiquitination of RIG-I and MDA5 at the same time.
It indicated that OTUD3 is a negative regulator in innate immunity mediated by RIG-I and MDA5.”
Prof. Wuhan Xiao, Institute of Hydrobiology, Chinese Academy of Sciences
During an antiviral innate immune response, unanchored K63 ubiquitin chains cause RIG-I and MDA5 to oligomerize, resulting in their activation. The researchers next looked at how OTUD3 influences RIG-I and MDA5 oligomerization. OTUD3 inhibits oligomerization and hinders viral RNA from binding to RIG-I and MDA5, according to their findings.
Image Credit: vchal/Shutterstock.com
The E3 ligases RNF135 and TRIM65 are thought to target RIG-I and MDA5 for K63-linked polyubiquitination, specifically. The researchers next performed ubiquitination experiments in 293T cells after transfecting RNF135 with RIG-I or TRIM65 with MDA5. Researchers also found that OTUD3 can prevent RNF135- and TRIM65-induced RIG-I and MDA5 K63-linked polyubiquitination.
The researchers employed mice and zebrafish as models to assess the relevance of OTUD3 in the host fighting RNA viral infections in vivo and found that OTUD3-deficient animals and zebrafish are more susceptible to RNA viral infection.
The impact of OTUD3 on DNA virus-triggered innate antiviral signaling was later discovered by the researchers.
What we found interesting is that OTUD3 is a positive regulator in response to DNA virus infection, and OTUD3 can interact with cGAS and catalyzed the removal of K48-linked polyubiquitin chains.”
Prof. Wuhan Xiao, Institute of Hydrobiology, Chinese Academy of Sciences
The researchers used mutation and ubiquitination experiments to further understand the mechanism of OTUD3 in DNA virus-triggered innate antiviral signaling. Researchers discovered that OTUD3 cleaves K48-linked polyubiquitination chains from Lys279 of cGAS. It then enhanced cGAS’ DNA-binding capabilities while also protecting it from degradation.
In addition, mice and zebrafish were employed to establish that OTUD3 controlled antiviral immune responses produced by DNA viruses in vivo.
This study discovered that depending on whether it is reacting to an RNA or DNA virus, OTUD3 has various functions in innate immunity, demonstrating the diversity of the host antiviral response.
Source:
Journal reference:
Cai, X., et al. (2022) Opposing effects of deubiquitinase OTUD3 in innate immunity against RNA and DNA viruses. Cell Reports. doi.org/10.1016/j.celrep.2022.110920.