Reviewed by Danielle Ellis, B.Sc.Jun 13 2022
To drive anabolic processes, epithelial ovarian cancer (EOC) is heavily reliant on the tricarboxylic acid (TCA) cycle and oxidative phosphorylation. S-palmitoylation is the process of adding the saturated 16-carbon fatty acid palmitate to a cysteine residue to produce a thioester linkage.
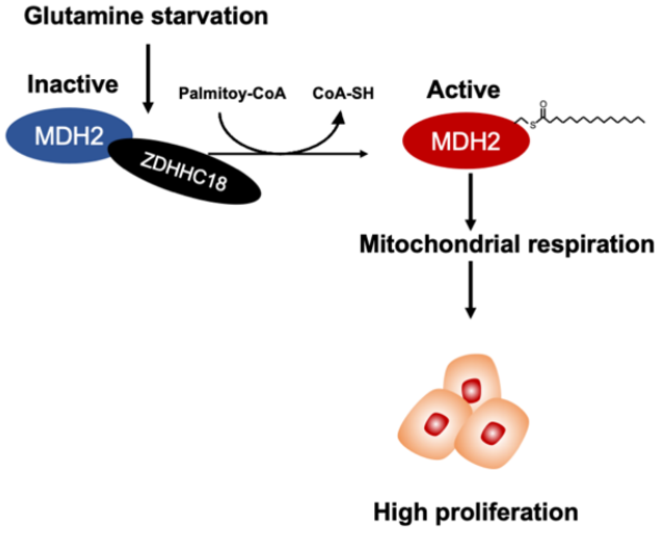 The model illustrating MDH2 C138 palmitoylation accelerates mitochondrial respiration and cell growth of ovarian cancer. Image Credit: ©Science China Press
The model illustrating MDH2 C138 palmitoylation accelerates mitochondrial respiration and cell growth of ovarian cancer. Image Credit: ©Science China Press
The hydrophobic property of target proteins is altered by S-palmitoylation, which regulates their cellular localization, trafficking, longevity, activity, and signalling.
Recently, a paper has been published in Science China-Life Sciences.
Malate dehydrogenase 2 (MDH2), a major enzyme in the TCA cycle, is palmitoylated at cysteine 138 (C138) residue, resulting in higher MDH2 activity, according to researchers. They then discovered that ZDHHC18 is an MDH2 palmitoyltransferase. By enhancing the interaction between ZDHHC18 and MDH2, glutamine deficiency promotes MDH2 palmitoylation.
In vitro and in vivo, silencing MDH2 inhibits mitochondrial respiration as well as ovarian cancer cell proliferation. Re-expression of wild-type MDH2, but not its palmitoylation-deficient C138S mutant, maintains mitochondrial respiration and rebuilds ovarian cancer cell proliferation and clonogenic capacity. The degree of palmitoylation of MDH2 in clinical cancer samples from patients with high-grade serous ovarian carcinoma is particularly high.
These findings demonstrate that ZDHHC18-mediated MDH2 palmitoylation maintains mitochondrial respiration and enhances the malignancy of ovarian cancer, suggesting that targeting ZDHHC18-mediated MDH2 palmitoylation in the cure of EOC could be beneficial.
Source:
Journal reference:
Pei, X., et al. (2022) Palmitoylation of MDH2 by ZDHHC18 activates mitochondrial respiration and accelerates ovarian cancer growth. Science China Life Sciences. doi.org/10.1007/s11427-021-2048-2.