Reviewed by Danielle Ellis, B.Sc.Jun 20 2022
Around 59,000 individuals are shockingly killed by the rabies virus each year, and several of them are children. Some victims, particularly children, discover they have been exposed after it is too late. Others are unable to undergo the rigorous rabies treatment regimen because it is not generally available and costs an average of $3,800, which would be unfathomable for most people around the globe.
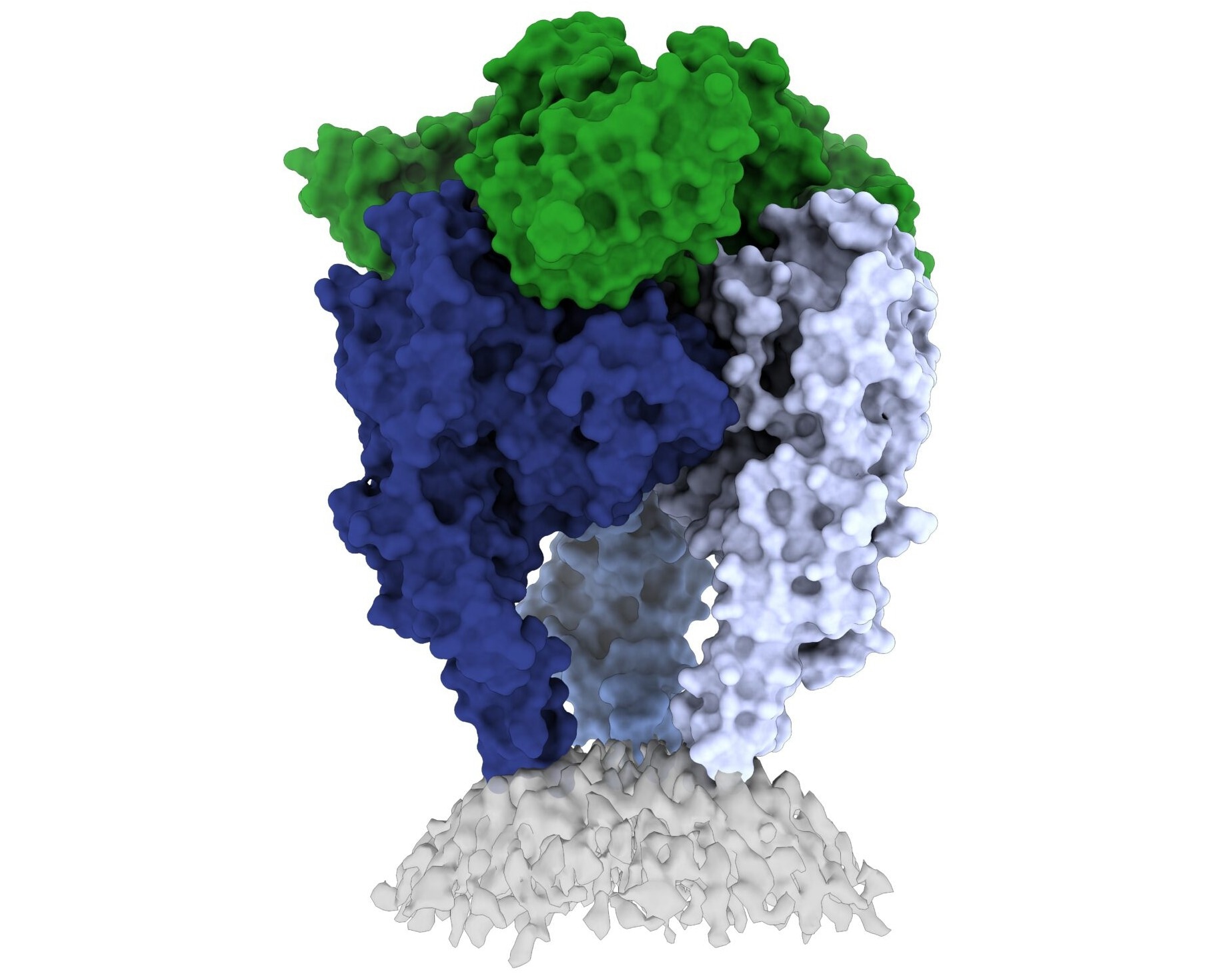
Image Credit: La Jolla Institute for Immunology
Treatments for rabies are substantially more expensive and difficult to provide than rabies immunizations. However, there is a serious drawback to the vaccinations:
Rabies vaccines don’t provide lifelong protection. You have to get your pets boosted every year to three years. Right now, rabies vaccines for humans and domestic animals are made from killed virus. But this inactivation process can cause the molecules to become misshapen—so these vaccines aren’t showing the right form to the immune system. If we made a better shaped, better structured vaccine, would immunity last longer?”
Erica Ollmann Saphire PhD, Professor, La Jolla Institute for Immunology
The road to improved vaccine design may have been found by Saphire and her colleagues, working with a group under the direction of Hervé Bourhy, DVM, PhD, professor at the Institut Pasteur.
The researchers present one of the first high-resolution views of the rabies virus glycoprotein in its weak “trimeric” form in a recent study, which was published in Science Advances.
The rabies glycoprotein is the only protein that rabies expresses on its surface, which means it is going to be the major target of neutralizing antibodies during an infection.”
Heather Callaway PhD, Study First Author and Postdoctoral Fellow, La Jolla Institute for Immunology
“Rabies is the most lethal virus we know. It is so much a part of our history—we’ve lived with its specter for hundreds of years,” states Saphire, who is also the President and CEO of LJI.
Saphire adds, “Yet scientists have never observed the organization of its surface molecule. It is important to understand that structure to make more effective vaccines and treatments—and to understand how rabies and other viruses like it enter cells.”
“This understanding is crucial for designing better vaccines and for developing treatments to cure rabid patients,” says Bourhy.
Rabies the shapeshifter
Although they do not fully understand why rabies vaccines do not offer long-term safety, researchers are aware that the disease’s shape-shifting proteins are a concern.
The rabies glycoprotein includes sequences that can unfurl and flip upward, when necessary, similar to a Swiss Army knife. The glycoprotein can alternate between pre-fusion and post-fusion states before fusing with a host cell. Additionally, it has the ability to disassemble, converting from a trimer structure (where three copies are bundled together) to a monomer (one copy by itself).
The ability to change shapes grants rabies a veil of invisibility. Human antibodies are developed to detect specific protein sites. When a protein changes to conceal or shift such locations, they are unable to follow along.
The latest research provides an important image of the ideal glycoprotein shape to address antibody defense.
Capturing the glycoprotein at last
Callaway spent three years stabilizing and freezing the trimeric version of the rabies glycoprotein. The glycoprotein assumes this “pre-fusion” state before infecting human cells.
Callaway discovered one location where the viral structure is susceptible to antibody attacks by combining the glycoprotein with a human antibody. Using advanced cryo-electron microscopy technology at LJI, the scientists next obtained a 3D image of the glycoprotein.
“This helps us to understand the mode of action of the antibodies neutralizing the virus,” comments Bourhy.
Scientists had never before noticed a number of important traits highlighted by the new 3D structure. It is significant that the structure accurately depicts two essential components of the virus structure, known as the fusion peptides. During infection, these two segments, which connect the glycoprotein’s bottom to the viral membrane, protrude into the target cell.
These sequences are extremely difficult to capture in a stable photograph. To try and get images of the glycoprotein, other rabies researchers had to chop them off.
By encapsulating the rabies glycoprotein in detergent molecules, Callaway found a solution to this issue.
That let us see how the fusion sequences are attached before they snap upward during infection.”
Erica Ollmann Saphire PhD, Professor, La Jolla Institute for Immunology
With a clearer understanding of the viral structure, researchers can now create vaccinations that instruct the body to produce antibodies that can attack the virus.
“Instead of being exposed to four-plus different protein shapes, your immune system should really just see one—the right one. This could lead to a better vaccine,” says Callaway.
Preventing a family of viruses
In addition to the billions of humans who may unintentionally come into contact with a rabid animal, Saphire expects that people who regularly interact with animals, such as veterinarians and wildlife workers, will benefit from better, wider immunity. Dogs, raccoons, bats, and skunks are just a few of the many creatures that can contract rabies, which is indigenous to all continents excluding Antarctica.
Vaccination against the entire lyssavirus genus, which includes rabies and other viruses that can travel between humans and other mammals, may also be possible as a result of this new research.
The following phase in this research is to take more pictures of the rabies virus and related viruses as well as antibodies that neutralize them. According to Callaway, researchers are aiming to solve a number of these structures, which could indicate shared antibody targets among lyssaviruses.
“Because we didn’t have these structures of the rabies virus in this conformational state before, it’s been hard to design a broad-spectrum vaccine,” says Callaway.
Source:
Journal reference:
Callaway, H. M., et al. (2022) Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science Advances. doi.org/10.1126/sciadv.abp9151.