A single gene that was previously discovered to be the main factor in a rare disease associated with epilepsy, autism, and developmental delay has been named as a key player in the development of healthy neurons.
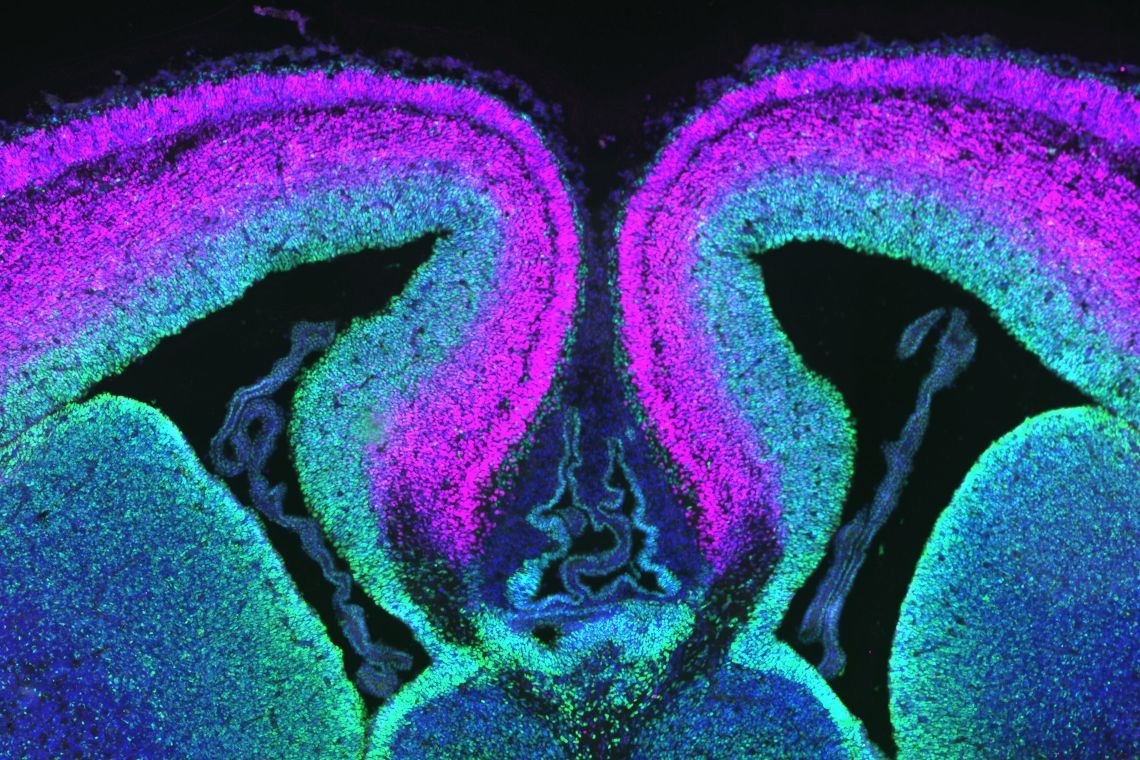 A cross-section of mouse cerebral cortex with progenitor cells labeled green and neurons in magenta. Image Credit: Debra Silver Lab, Duke University
A cross-section of mouse cerebral cortex with progenitor cells labeled green and neurons in magenta. Image Credit: Debra Silver Lab, Duke University
According to Duke scientists, the DDX3X gene produces a cellular apparatus called a helicase, whose function is to break open the hairpins and cul-de-sacs of RNA so that the cell’s equipment for building proteins can read their instructions. Since this gene is located on the X chromosome, males and females both have one copy of the gene.
If you remove both copies of the gene in a female mouse that results in a massive microcephaly where the brains are severely reduced in size.”
Debra Silver, PhD, Associate Professor, Molecular Genetics and Microbiology, Duke School Of Medicine
Silver also directed the research team. “But the removal of a single copy is probably more closely mimicking what’s happening in human patients,” Silver said.
To put it differently, the severity of the disease depends on how severely the mutations impact the synthesis of helicases. This is because the abnormalities brought on by defective DDX3X are dose-dependent. The results are published in the open-access journal eLife on June 28th, 2022.
When DDX3X is changed by a mutation in initial development, “you don’t get as many neurons over time because this gene is required for the production of neurons from progenitor cells,” Silver said. “And it is also helping the progenitors to divide properly.”
A mutant DDX3X may cause the division of a nerve precursor cell to take more time than the typical 15 hours, according to Silver.
“And what that means over time, if these neural precursors are taking too long to divide, is you fall behind, and the brain doesn’t develop properly.”
An earlier study by the same team, published in March 2020, showed that nearly half of the DDX3X mutations destroyed the gene, while the other half just made it function more inefficiently. The study used genetic samples from 107 developmentally impaired children throughout the world.
One to three percent of intellectual disorders in females are now thought to be caused by DDX3X mutations—however, these mutations are virtually invariably “de novo,” meaning they occurred spontaneously during a developmental phase rather than being transmitted from the parents.
Since almost all of the youngsters in the earlier trial were male, scientists assumed that losing DDX3X in males would be fatal because they only have one copy of the gene. However, in this study, Silver’s team found that DDX3Y, a partner gene found on the Y chromosome of males, could perform some of the gene’s functions.
To carry out this research, Mariah Hoye’s group at Silver’s University created a novel method for profiling all newly produced proteins produced by progenitor cells in the brains of living animals. This method could help us understand how protein synthesis occurs in the brain, Hoye added.
According to Silver, several of the RNAs whose translation is hindered by DDX3X damage play a part in the growth of the brain.
So it’s helping us to discover what I would call a network of RNAs whose translation depends on this gene. And it starts to give us clues as to how, molecularly, DDX3X may be disrupting brain development.”
Debra Silver, PhD, Associate Professor, Molecular Genetics and Microbiology, Duke School Of Medicine
DDX3X has also been linked to innate immune responses, certain cancer development, and neurodegeneration. According to Silver, comprehending the cellular mechanisms and molecular targets of DDX3X in the growing brain may provide insight into the causes of a variety of illnesses.
“We know of more than 800 families worldwide who have been diagnosed with DDX3X syndrome,” Silver said. “This is definitely an important gene, with likely hundreds of mutations. There are tons to learn about how DDX3X controls brain development.”
“We hope this research can improve an understanding of the basis for DDX3X syndrome and related disorders,” Silver said. “In the longer term, this may help contribute to the development of therapies.”
Source:
Journal reference:
Hoye, M. L., et al. (2022) Aberrant cortical development is driven by impaired cell cycle and translational control in a DDX3X syndrome model. eLife. doi.org/10.7554/eLife.78203.