The cancer type cholangiocarcinoma, often known as bile duct cancer, is characterized by a high fatality rate. Most bile duct tumors are usually already terminal at the time of diagnosis. Therefore, there is an urgent need for techniques for the early detection of bile duct cancer.
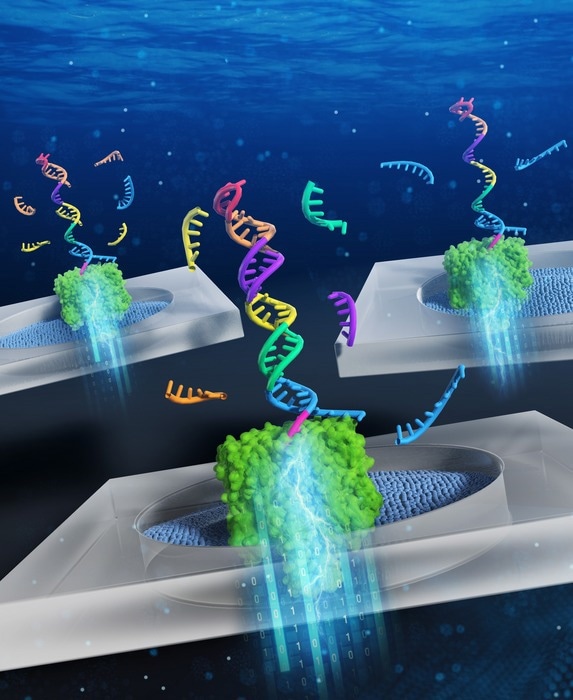 Pattern recognition of miRNA expression using DNA computing and nanopore decoding. Image Credit: Ryuji Kawano, Tokyo University of Agriculture and Technology
Pattern recognition of miRNA expression using DNA computing and nanopore decoding. Image Credit: Ryuji Kawano, Tokyo University of Agriculture and Technology
Liquid biopsy, which involves taking a sample of non-solid biological tissue like blood, is gaining popularity as a quick and painless way to detect cancer. A liquid blood biopsy just needs a few milliliters of blood, with no risk to the patient, in contrast to standard biopsies that frequently require surgery and general anesthesia.
Following a sample, the blood is examined for particular indicators that suggest the existence of malignant tissue.
For instance, unique microRNA (miRNA) patterns, which are short non-coding strands of RNA, have been linked to various forms of cancer and can be utilized to accurately identify tumors from liquid biopsies. It is difficult to identify miRNA in blood samples due to their low concentration.
Scientists at the Tokyo University of Agriculture and Technologies, using DNA computing technology, have created a novel technique for cancer detection of miRNA patterns. With small concentrations of the target biomarkers in liquid biopsies, the new approach has the potential to be a useful tool for straightforward and initial cancer diagnosis. On June 26, 2022, the results were released in the peer-reviewed journal JACS Au.
DNA computing uses the biochemical reactions of the information-encoding DNA molecules to solve problems based on formal logic, in the same way that normal computers do.”
Ryuji Kawano, Study Corresponding Author and Professor, Tokyo University of Agriculture and Technology
“In this case, a diagnostic DNA molecule was designed to be able to bind five different kinds of miRNA associated with bile duct cancer. In the process of binding the miRNA molecules, the diagnostic DNA converts the expression pattern of the miRNAs into the information contained in the form of a nucleic acid structure,” Kawano added.
The researchers employ a technique called nanopore decoding to read this data. This process involves passing the DNA via a nanoscale hole, or “pore.” The molecule will block the passage of electrical current through the pore as it moves through it.
The features of the passing molecule can then be inferred from measurements of these changes in the current through the pore. The attached miRNAs will “unzip” from the DNA in the case of the diagnostic DNA, leading to a current inhibition with a recognizable amplitude and duration.
Even from clinical samples with incredibly low amounts of miRNA, the researchers were able to identify cancer-specific expression patterns by statistical analysis of the unzipping data of the miRNA patterns.
This is a significant advancement in the field of nanopore diagnostics since it was previously believed that nanopore measurements could not identify nucleic acids at these low concentrations, undermining their usefulness for clinical applications.
Source:
Journal reference:
Takeuchi, N., et al. (2022) Pattern Recognition of microRNA Expression in Body Fluids Using Nanopore Decoding at Subfemtomolar Concentrations. JACS Au. doi.org/10.1021/jacsau.2c00117.