The use of attenuated, live viruses as vaccines is a potential method to minimize the effects of viral infectious diseases, such as influenza.
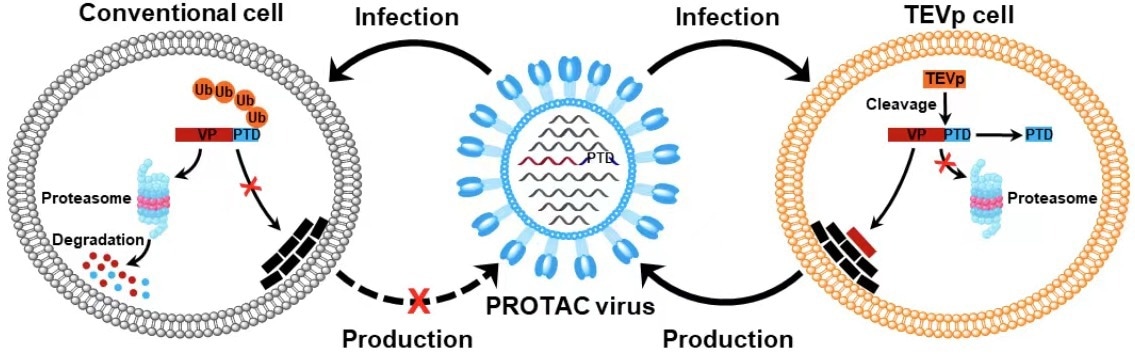 Schematic illustration of the generation of PROTAC viruses (VP, viral protein; Ub, ubiquitin). Image Credit: SI Longlong.
Schematic illustration of the generation of PROTAC viruses (VP, viral protein; Ub, ubiquitin). Image Credit: SI Longlong.
Traditional live-attenuated virus vaccines have, however, frequently been shown to be ineffective due to poor immunogenicity, security issues, or cumbersome production procedures. Conventional influenza vaccinations have an additional obstacle from immunological escape brought on by the fast development of the virus.
A novel live-attenuated influenza vaccination technique has recently been proposed by a research team led by Prof. SI Longlong from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences. This strategy involves creating proteolysis-targeting chimeric (PROTAC) influenza. By using the natural ubiquitin-proteasome system of host cells to break down viral proteins, a virus can be used as a live-attenuated vaccine.
On July 4th, 2022, the findings were released in Nature Biotechnology.
Given that virally encoded proteins are necessary for virus replication, manipulating viral protein stability by making use of the host cell’s protein degradation machinery may provide a potential method to turn the viral life cycle on and off for vaccine development. Thus, by attaching a conditionally detachable proteasome-targeting domain (PTD) to influenza viral proteins, the researchers created proteolysis-targeting chimeric (PROTAC) viruses.
A tobacco etch virus cleavage site (TEVcs) linker and a proteasome-targeting peptide was included in the design of the PTD. However, the TEVcs linker may be selectively cleaved by the tobacco etch virus protease (TEVp) to remove the viral proteins from the PTD, protecting them from destruction. It was utilized to specifically induce proteasomal degradation of viral proteins of interest.
In order to introduce the conditionally removable PTD, the researchers engineered the influenza A virus genome in TEVp-expressing stable cell lines designed for virus production. This resulted in completely infective PROTAC viruses that were live-attenuated by the host protein deterioration machinery upon infection.
The PROTAC viruses were suitably attenuated in mouse and ferret models, yet still managed to elicit strong and widespread humoral, mucosal, and cellular protection. They, thus, provide extensive defense against both homologous and heterologous viral threats.
This PROTAC vaccine technology could also be useful for generating live-attenuated vaccines against other types of pathogens.”
Prof. Longlong Si, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences
Source:
Journal reference:
Si, L., et al. (2022) Generation of a live attenuated influenza A vaccine by proteolysis targeting. Nature Biotechnology. doi.org/10.1038/s41587-022-01381-4.