Mitochondria are generally known as the powerhouse of the cell, however, growing research indicates that they may potentially be involved in inflammation. Researchers from the Salk Institute and UC San Diego looked at human blood cells and found a surprising connection between mitochondria, inflammation, and the genes DNMT3A and TET2, which usually help control blood cell growth but are linked to a higher risk of atherosclerosis when mutated.
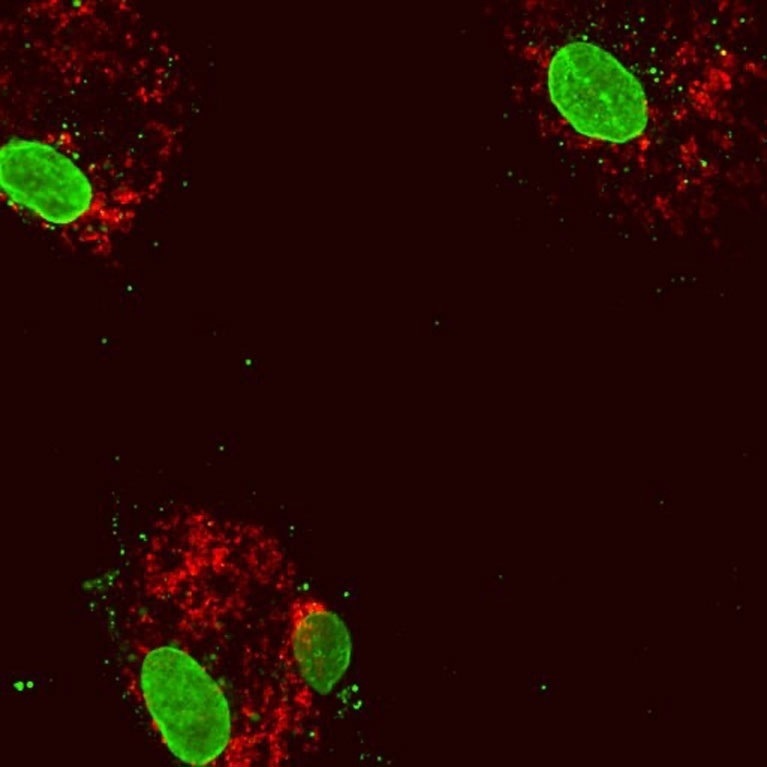 Human blood cells following reduced expression of the gene DNMT3A. The cell nuclei (large green structures) inside the cytoplasmic protein (red). Some mitochondrial DNA (small green dots) has escaped into the cytoplasm, inducing an inflammatory response. Image Credit: Isidoro Cobo of UC San Diego
Human blood cells following reduced expression of the gene DNMT3A. The cell nuclei (large green structures) inside the cytoplasmic protein (red). Some mitochondrial DNA (small green dots) has escaped into the cytoplasm, inducing an inflammatory response. Image Credit: Isidoro Cobo of UC San Diego
The study was published on August 2nd, 2022, in the Immunity journal.
We found that the genes DNMT3A and TET2, in addition to their normal job of altering chemical tags to regulate DNA, directly activate expression of a gene involved in mitochondrial inflammatory pathways, which hints at a new molecular target for atherosclerosis therapeutics.”
Gerald Shadel, Professor and Co-Senior Author, Salk Institute for Biological Studies
Shadel was also the director of the San Diego Nathan Shock Center of Excellence in the Basic Biology of Aging.
The investigation into the functions of DNMT3A and TET2 mutations in clonal hematopoiesis—the process by which altered immature blood cells give rise to a population of mature blood cells with the same mutations—led UC San Diego researchers to discover a particular inflammatory response.
They observed that DNMT3A and TET2 deficiency in blood cells, which play a significant role in the inflammatory response and advanced atherosclerosis, were also connected to aberrant inflammatory signaling.
However, it was unclear how the DNMT3A and TET2 genes were related to inflammation and perhaps atherosclerosis.
The problem was we couldn’t work out how DNMT3A and TET2 were involved because the proteins they code do seemingly opposite things regarding DNA regulation. Their antagonistic activity led us to believe there may be other mechanisms at play. This prompted us to take a different approach and contact Shadel, who had uncovered the same inflammatory pathway years earlier while examining responses to mitochondrial DNA stress.”
Christopher Glass, Study Co-Senior Author and Professor, University of California San Diego School of Medicine
A specific subset of the cell’s DNA must be correctly structured and condensed inside the mitochondria to maintain a normal operation. Shadel’s team to study the effects of mitochondrial DNA stress previously used TFAM, a gene that ensures mitochondrial DNA is packaged properly.
They discovered that mitochondrial DNA leaves the mitochondria and enters the interior of the cell when TFAM levels are decreased. The same molecular alarm that alerts the cell to the presence of a bacterial or viral invasion is triggered by this, and a protective molecular pathway that encourages inflammation is also triggered.
To comprehend why DNMT3A and TET2 mutations caused inflammatory responses akin to those seen during mitochondrial DNA stress, researchers from the Glass and Shadel laboratories collaborated. The scientists examined cells from patients with normal cells, those with function loss mutations in DNMT3A or TET2 expression, and those with atherosclerosis using genetic engineering methods and cell imaging.
They discovered that experimentally suppressing the expression of DNMT3A or TET2 in healthy blood cells led to an upped inflammatory response, which was also observed in blood cells with loss-of-function mutations and blood cells from people with atherosclerosis.
Surprisingly, lower TFAM expression caused by low DNMT3A and TET2 expression in blood cells results in aberrant mitochondrial DNA packaging, which triggers inflammation because of released mitochondrial DNA.
“We discovered that DNMT3A and TET2 mutations prevent their ability to bind and activate the TFAM gene. Missing or reducing this binding activity leads to mitochondrial DNA release and overactive mitochondrial inflammation response, and we believe this may exacerbate plaque buildup in atherosclerosis,” says first author Isidoro Cobo, a postdoctoral researcher in the Glass lab at UC San Diego.
“It’s very exciting to see our discovery on TFAM depletion causing mitochondrial DNA stress and inflammation now has direct relevance for a disease like atherosclerosis. Ever since we revealed this pathway, there has been an explosion of interest in mitochondria being involved in inflammation and many reports linking mitochondrial DNA release to other clinical contexts,” says Shadel, who holds the Audrey Geisel Chair in Biomedical Science.
For many other disorders, existing medications target the signaling pathways involved in inflammation. In individuals with TET2A and DNMT3A mutations, inhibiting pathways that increase atherosclerosis may be the basis for new therapeutic approaches, according to Glass and Shadel. The experts will then carry out more research on this route and look into the role of mitochondrial DNA in other human diseases and aging.
Source:
Journal reference:
Cobo, I., et al. (2022) DNA methyltransferase 3 alpha and TET methylcytosine dioxygenase 2 restrain mitochondrial DNA-mediated interferon signaling in macrophages. Immunity. doi.org/10.1016/j.immuni.2022.06.022.