The Gerlich Group at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences (IMBA) has identified a molecular process that gives chromosomes in dividing human cells unique physical characteristics that permit faithful transmission to the progeny.
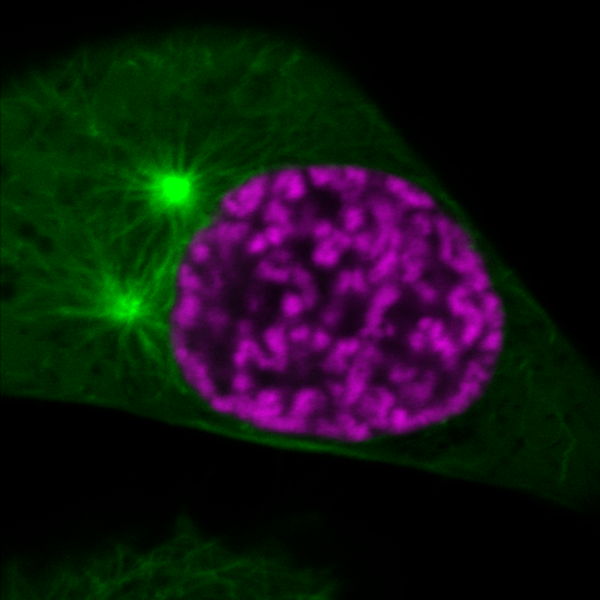 Organization of mitotic chromosomes (magenta) and spindle microtubules (green) at an early phase of cell division. Shortly after what’s shown in the image, the microtubules will invade the nuclear space. However, chromatin compaction regulated by histone acetylation will prevent the perforation of the chromosomes by microtubules. Image Credit: ©Gerlich/IMBA.
Organization of mitotic chromosomes (magenta) and spindle microtubules (green) at an early phase of cell division. Shortly after what’s shown in the image, the microtubules will invade the nuclear space. However, chromatin compaction regulated by histone acetylation will prevent the perforation of the chromosomes by microtubules. Image Credit: ©Gerlich/IMBA.
The team demonstrated how chromosomes can develop a strong surface boundary by chemical alteration, preventing them from being pierced by spindle apparatus microtubules. The results are published in the Nature journal.
Exactly one genome copy must be transferred from the parent cell to each of the two daughter cells during cell division. Extremely lengthy chromosomal DNA molecules must be packaged into discrete bodies for faithful genome segregation for the mitotic spindle, a filament structure composed of thousands of microtubules, to transport them effectively.
The recent research from the Gerlich Research Group at the IMBA sheds light on how mitotic chromosomes withstand the continual pushing and pulling stresses produced by the microtubules.
Amidst this complex system, the distinct physical properties are conferred to the chromosomes by changing the levels of histone acetylation, a chemical modification within the chromatin fiber.”
Daniel Gerlich, Group Leader, Institute of Molecular Biotechnology of the Austrian Academy of Sciences
The chromatin fibers in dividing cells are folded into loops by a big protein complex called condensin, according to earlier research. Condensin’s function could not, however, account for why chromosomes resemble pointy, dense structures rather than a loose, bottlebrush-like shape.
Although some studies have suggested that histone acetylation may play a role in controlling the degree of compaction during cell division, the interaction between histone acetylation and condensin as well as its functional significance remains uncertain.
With our work, we are now able to conceptually disentangle the two mechanisms.”
Daniel Gerlich, Group Leader, Institute of Molecular Biotechnology of the Austrian Academy of Sciences
To further understand their precise effects, the team changed the concentrations of condensin and histone acetylation. Condensin removal reduced the resistance of chromosomes to pulling forces and altered their elongated form in dividing cells, but had no effect on the degree of compaction.
Combining condensin depletion with a method that increases histone acetylation levels led to severe chromatin decompaction in dividing cells and microtubule perforation of chromosomes.
The investigators proposed that during most of the cell cycle (when it is relatively strongly acetylated), chromatin is organized as a swelling gel and that during cell division, when acetylation levels generally decline, this gel compacts to an insoluble state.
They subsequently created an experiment to examine the solubility of chromatin by chopping up mitotic chromosomes. When the level of acetylation was raised, the liquid chromatin droplets generated by the mitotic chromosome pieces disintegrated into the cytoplasm.
The findings are consistent with a hypothesis in which, during mitosis, a worldwide decrease in chromatin acetylation creates an immiscible chromatin gel with a distinct phase boundary, providing a physical foundation for resistance to microtubule perforation.
The team discovered that immiscible chromatin forms a structure dense in negative charge that excludes negatively charged macromolecules and microtubules through additional experiments using pure chromatin that was reconstituted in vitro and by probing chromatin access by various soluble macromolecules.
Our study shows how DNA looping by the condensin complex cooperates with a chromatin phase separation process to build mitotic chromosomes that resist both pulling and pushing forces exerted by the spindle. The deacetylation of histones during cell division hence confers unique physical properties to chromosomes that are required for their faithful segregation.”
Daniel Gerlich, Group Leader, Institute of Molecular Biotechnology of the Austrian Academy of Sciences
Source:
Journal reference:
Schneider, M. W. G., et al. (2022) A mitotic chromatin phase transition prevents perforation by microtubules. Nature. doi.org/10.1038/s41586-022-05027-y.