The task of battling cancer cells can fatigue T cells employed in immunotherapy treatments, or they could shut down once they penetrate tumors. Researchers at the Gladstone Institutes and UC San Francisco have strengthened the therapeutic cells’ resistance via a CRISPR-based edit on their genomes.
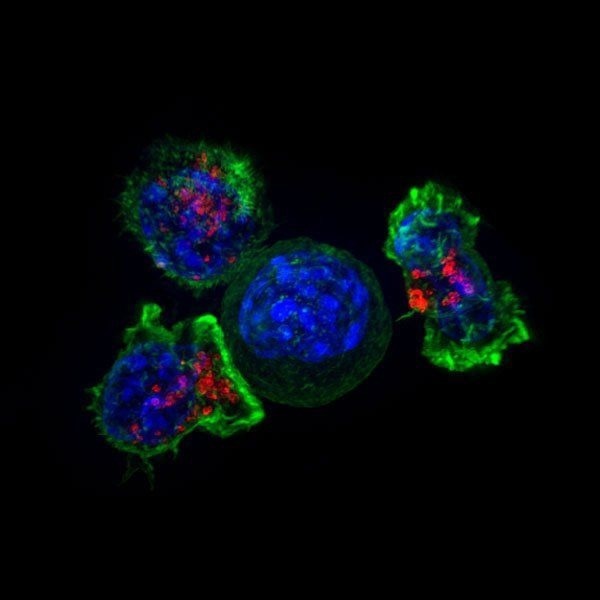 Microscopic image of a group of killer T cells (green and red) surrounding a cancer cell (blue, center). Image Credit: Alex Ritter, Jennifer Lippincott Schwartz, and Gillian Griffiths, National Institutes of Health
Microscopic image of a group of killer T cells (green and red) surrounding a cancer cell (blue, center). Image Credit: Alex Ritter, Jennifer Lippincott Schwartz, and Gillian Griffiths, National Institutes of Health
The discovery could assist in overcoming a significant obstacle impeding these effective medicines’ ability to control both solid and liquid cancers.
We have succeeded in engineering better, stronger, longer-lived T cells that we think will improve treatment of both blood and solid cancers.”
Alex Marson, Study Co-Leader and Professor, Medicine, University of California San Francisco
The study, which was headed by Alex Marson, MD, PhD, and cancer biologist Alan Ashworth, PhD, FRS, was published in Nature on August 24th, 2022.
Marson added, “It is an example of how we are using the power of CRISPR to accelerate the design of improved T-cell therapies.”
Marson is also the director of the Gladstone-UCSF Institute of Genomic Immunology, a collaboration between the two institutions that aims to develop new cell-based immunotherapies by combining a variety of state-of-the-art genomic technologies.
These genomic innovations are creating avenues to tackle the challenges of developing highly effective and precisely targeted immunotherapies. The capacity to direct cell behavior by manipulating the genome will likely lead to transformational changes in the treatment of many diseases.”
Alan Ashworth, Study Co-Leader and President, Helen Diller Family Comprehensive Cancer Center, University of California San Francisco
The fact that cancers reside in an environment that inhibits T cells and other immune cells, allowing the tumor to form and grow, is one of the primary obstacles to the development of highly effective cancer immunotherapies.
Therapeutic T cells, which are created from a patient's own T cells and are designed to recognize and destroy tumor cells, frequently become worn out or dysfunctional while battling this environment and are unable to kill the cancer cells.
By knocking out one individual gene, we have created cells that are not just potent tumor cell killers but also more persistent killers over a long period of time.”
Julia Carnevale, Study Corresponding Author and Assistant Professor, Medicine, University of California San Francisco
She recently established her own lab in the Department of Medicine at UCSF, where she is working to develop innovative methods for creating improved cell therapies for the treatment of cancer.
An unexpected target
The team discovered a small number of candidates that could make the T cells resistant to significant elements of the immune-suppressive microenvironment frequently found in tumors by using a set of CRISPR screens that permitted them to turn off each gene in the genome, one at a time, in a pool of human T cells.
Carnevale, who is also a Gladstone affiliate investigator, and co-corresponding author Eric Shifrut, PhD, were particularly interested in one gene called RASA2 because it had never been linked to immune cell function before.
This was uncharted T-cell biology. By focusing on RASA2, we wanted to find out whether controlling expression of the gene in human T cells might make them more sensitive immunotherapy agents.”
Eric Shifrut, Co-Corresponding Author and Assistant Professor, Tel Aviv University
The team produced T cells with the RASA2 gene knocked out using models developed by corresponding authors Giedre Krenciute, PhD, of St. Jude Children’s Research Hospital, and Justin Eyquem, PhD. After that, they put these T cells through a number of “stress tests” by repeatedly exposing them to cancer cells and simulations of the tumor microenvironment.
The effectiveness of these cells was compared with that of the original therapeutic T cells that still had a functional RASA2 gene. The cells with RASA2 knocked out remained extraordinarily persistent long after the original cells had lost their capacity to combat malignancy.
Testing on various engineered T cell types in which the scientists blocked RASA2 yielded consistent findings, as did testing on cells from numerous different human donors, models of both liquid and solid cancer, and engineered T cells from diverse types of engineered T cells.
Carnevale added, “The knockout cells could just keep killing. It seems as if we found the brake in the system and when we take it off, we unleash the potential of these therapeutic cells.”
Improving existing therapies
Shifrut stated now that Carnevale has a viable target, she and her colleagues are researching it in preclinical models to streamline its effectiveness and evaluate its safety, an important component of the research.
Shifrut remarked, “We want to ensure that when we remove RASA2’s braking effect, the T cells only recognize and attack cancer cells, and not healthy cells.”
The researchers are building the framework for a clinical trial by merging several advanced technologies with RASA2 deletion, to augment an already-existing T-cell therapy, in partnership with other labs at the institute.
“Even in the best-case scenarios, immunotherapy treatments don’t work for all patients and there are many examples of relapse. If we can push the boundary of this therapeutic approach by figuring out the right ways to rewire T cells, that would be really exciting,” added Carnevale.
The unprejudiced and rigorous CRISPR screening procedure, according to Carnevale and Shifrut, was responsible for identifying this unknown gene and expanding the reach of investigations like this.
Marson concluded, “This study taught us that RASA2 has roles in immunology that had previously been unexplored. Similar systematic studies, using CRISPR to look at every gene in the genome, will not only accelerate design of cancer therapies, but should also aid in designing cellular medicines that are better at treating all kinds of conditions, from autoimmune disorders to infectious disease.”
NIH funds 1K08CA252605-01, R01NS106379-02, R01CA173750, R01NS121249, K99CA256262, P30CA021765, 1S10OD010786-01, and P30CA082103 and Cancer Research Institute, Parker Institute for Cancer Immunotherapy, and other institutions and philanthropy supported the study.
Source:
Journal reference:
Carnevale, JS, et al (2022) RASA2 ablation in T cells boosts antigen sensitivity and long-term function. Nature. doi:10.1038/s41586-022-05126-w