Reviewed by Danielle Ellis, B.Sc.Sep 22 2022
For the neuronal cell types’ categorization as more complicated creatures, the genetic fingerprint of Nematostella vectensis—the sea anemone—reveals that the members of this evolutionarily very ancient animal phylum employ identical gene cascades. During the lifetime of an anemone, such genes are also accountable for the complete cell balance in the organism. A group of developmental biologists headed by Ulrich Technau of the University of Vienna published the findings in “Cell Reports.”
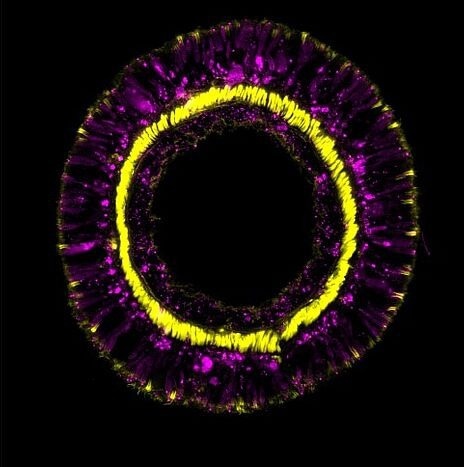 Cross-section through a tentacle of a transgenic sea anemone showing differentiation products of the SoxC cell population (magenta) and retractor muscles (yellow). Image Credits: Andreas Denner
Cross-section through a tentacle of a transgenic sea anemone showing differentiation products of the SoxC cell population (magenta) and retractor muscles (yellow). Image Credits: Andreas Denner
Nearly all animals are made of millions or billions of cells that gather together in complicated ways to build particular tissues and organs, which comprise several varied types of cells—for example, different varieties of gland cells or neurons. It is not well understood how this key balance of varied cell types arises, how it is controlled, and whether varied cell types of varied animals have a shared origin.

Image Credit: John A. Anderson/Shutterstock.com
Single-cell fingerprint leads to common ancestors
The study team headed by Ulrich Technau, an Evolutionary Developmental Biologist, who is also the head of the Single Cell Regulation of Stem Cells (SinCeReSt) research platform at the University of Vienna, has decoded the evolution and diversity of all gland and nerve cell types and their evolving origins in the sea anemone Nematostella vectensis.
In order to attain this, they employed single-cell transcriptomics, which is a method that has transformed evolutionary and biomedicine biology in the past decade.
With this, entire organisms can be resolved into single cells—and the entirety of all currently expressed genes in each individual cell can be decoded. Different cell types fundamentally differ in the genes they express. Therefore, single-cell transcriptomics can be used to determine the molecular fingerprint of each individual cell.”
Julia Steger, Study First Author, University of Vienna
Cells with an overlapping fingerprint were gathered in the study. This enabled the researchers to categorize defined cell types or cells in development’s transitional stages—each with unique expression combinations. It also enabled scientists to find the common progenitor and stem cell populations of the varied tissues.
Opposing to previous expectations, researchers found that glandular cells, neurons, and other sensory cells arise from one common progenitor population, which can be tested by genetic labeling in living organisms. This could specify a very old progressive relationship between gland cells and neurons.
Ancient gene in constant use
In the progression of these common ancestor cells, one gene performs an exceptional role. In all precursor cells of gland cells, neurons, and cnidocytes, SoxC is expressed and is important for the development of all these cell types, as the authors were also capable of revealing in knockout experiments.
Interestingly, this gene is no stranger: It also plays an important role in the formation of the nervous system in humans and many other animals, which, together with other data, shows that these key regulatory mechanisms of nerve cell differentiation seem to be conserved across the animal kingdom.”
Ulrich Technau, Head and Evolutionary Developmental Biologist, Single Cell Regulation of Stem Cells (SinCeReSt), University of Vienna
The authors also discovered that, in sea anemones, the neuron development’s genetic processes are regulated from the embryo to the grown-up organism by comparing different stages of life, consequently contributing to the neurons’ balance throughout the lifecycle of Nematostella Vectensis.
This is noteworthy because sea anemones, unlike humans, can substitute damaged or missing neurons during their lifecycle. For further studies, this brings up questions on how the sea anemone succeeds in maintaining these mechanisms that occur in the embryonic stage into the grown-up organism in a controlled manner in more complex animals.
Source:
Journal reference:
Steger, J., et al. (2022) Single cell transcriptomics identifies conserved regulators of neuroglandular lineages. Cell Reports. doi.org/10.1016/j.celrep.2022.111370.