Reviewed by Danielle Ellis, B.Sc.Sep 26 2022
According to a new study, tiny nets made of DNA strands can capture the spike protein of the virus that causes COVID-19, illuminating it for a quick yet accurate diagnostic test and preventing it from infecting cells, suggesting a new potential path to antiviral treatment.
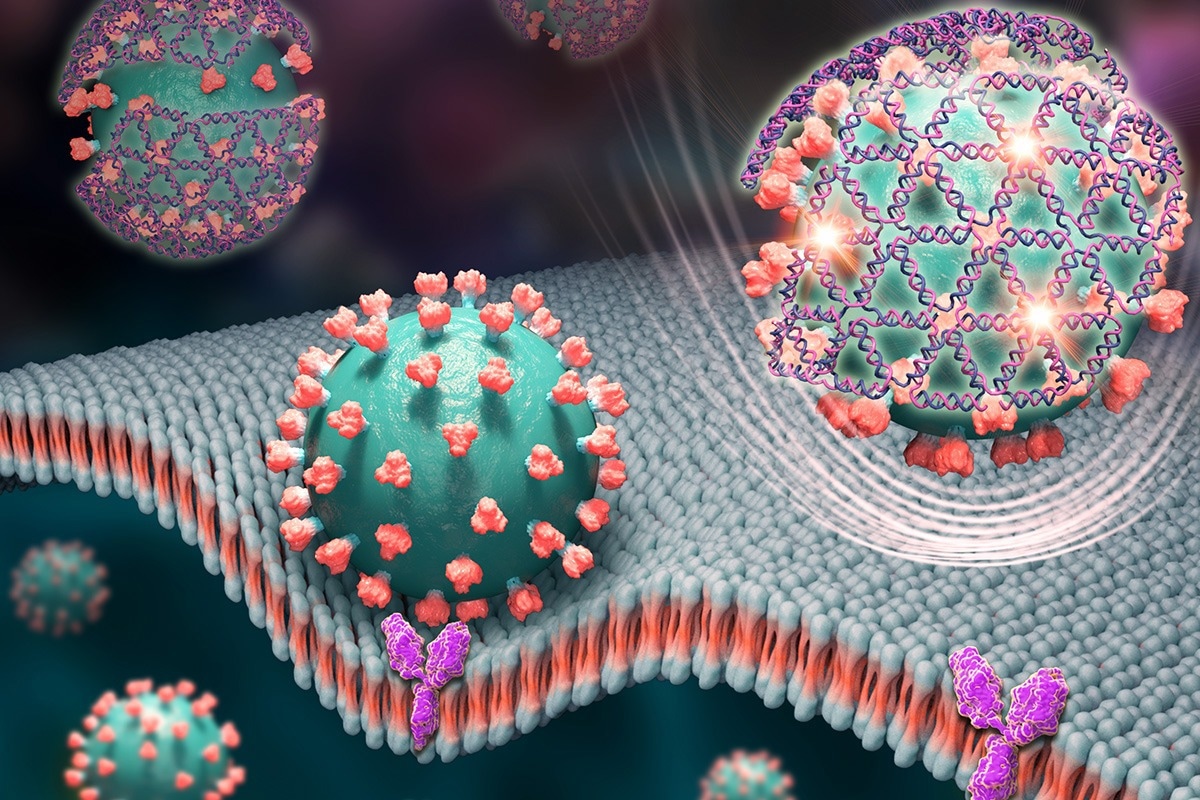 Tiny nets woven from DNA strands cover the spike proteins of the virus that causes COVID-19 and give off a glowing signal in this artist’s rendering. Image Credit: Xing Wang
Tiny nets woven from DNA strands cover the spike proteins of the virus that causes COVID-19 and give off a glowing signal in this artist’s rendering. Image Credit: Xing Wang
In a study published in the Journal of the American Chemical Society, researchers from the University of Illinois Urbana-Champaign and associates proved the DNA nets’ capacity to recognize and obstruct COVID-19 in human cell cultures.
This platform combines the sensitivity of clinical PCR tests and the speed and low cost of antigen tests. We need tests like this for a couple of reasons. One is to prepare for the next pandemic. The other reason is to track ongoing viral epidemics—not only coronaviruses, but also other deadly and economically impactful viruses like HIV or influenza.”
Xing Wang, Study Leader and Professor, Bioengineering and Chemistry, University of Illinois
DNA can be folded into specialized nanoscale structures that can perform functions or selectively attach to other structures, much like proteins can. DNA is well recognized for its genetic qualities, but it can also be folded into these structures.
The coronavirus spike protein, which protrudes from the virus’s surface and interacts with receptors on human cells to infect them, is targeted by the DNA nets created by the Illinois team. Once bound, the nets emit a fluorescent signal that takes about 10 minutes for a cheap handheld tool to read.
The researchers showed that their DNA nets successfully targeted the spike protein and could detect the virus at very low levels, which is equivalent to the sensitivity of gold-standard PCR tests that can detect the virus’ genetic material but can take a day or more to produce results from a clinical lab.
According to Wang, there are various benefits to the method. Users only need to mix the sample with the solution and examine the results; it requires no extra equipment or preparation and can be done at room temperature. In their investigation, the researchers calculated that the approach would cost $1.26 for each test.
Wang added, “Another advantage of this measure is that we can detect the entire virus, which is still infectious, and distinguish it from fragments that may not be infectious anymore.”
In addition to helping patients and doctors determine their level of infectiousness, this could significantly enhance community-level modeling and tracking of active outbreaks, such as those spread by wastewater.
Furthermore, the DNA nets prevented the virus from spreading in living cell cultures, and the antiviral activity grew as the DNA net scaffold’s size did. This suggests that DNA structures could have therapeutic value, according to Wang.
“I had this idea at the very beginning of the pandemic to build a platform for testing, but also for inhibition at the same time. Lots of other groups working on inhibitors are trying to wrap up the entire virus, or the parts of the virus that provide access to antibodies. This is not good, because you want the body to form antibodies. With the hollow DNA net structures, antibodies can still access the virus,” Wang further stated.
Wang said that the DNA net platform can be modified to work with additional viruses and even multiplexed to allow for the detection of numerous viruses by a single test.
Wang stated, “We are trying to develop a unified technology that can be used as a plug-and-play platform. We want to take advantage of DNA sensors’ high binding affinity, low limit of detection, low cost, and rapid preparation.”
Through the Rapid Acceleration of Diagnostics program, the National Institutes of Health provided funding for this research. The RADx program will be used by the researchers to continue exploring and advancing clinical applications for the DNA net platform.
Additionally, Wang is affiliated with Illinois’ Carl R. Woese Institute for Genomic Biology and Holonyak Micro and Nanotechnology Lab.
Source:
Journal reference:
Chauhan, N., et al. (2022) Net-Shaped DNA Nanostructures Designed for Rapid/Sensitive Detection and Potential Inhibition of the SARS-CoV-2 Virus. Journal of the American Chemical Society. doi:10.1021/jacs.2c04835