Reviewed by Danielle Ellis, B.Sc.Oct 6 2022
An RNA-based editing tool has been developed by Duke University researchers, which targets specific cells rather than genes. Any cell type can be precisely targeted, and any protein of interest can be added only to that cell type.
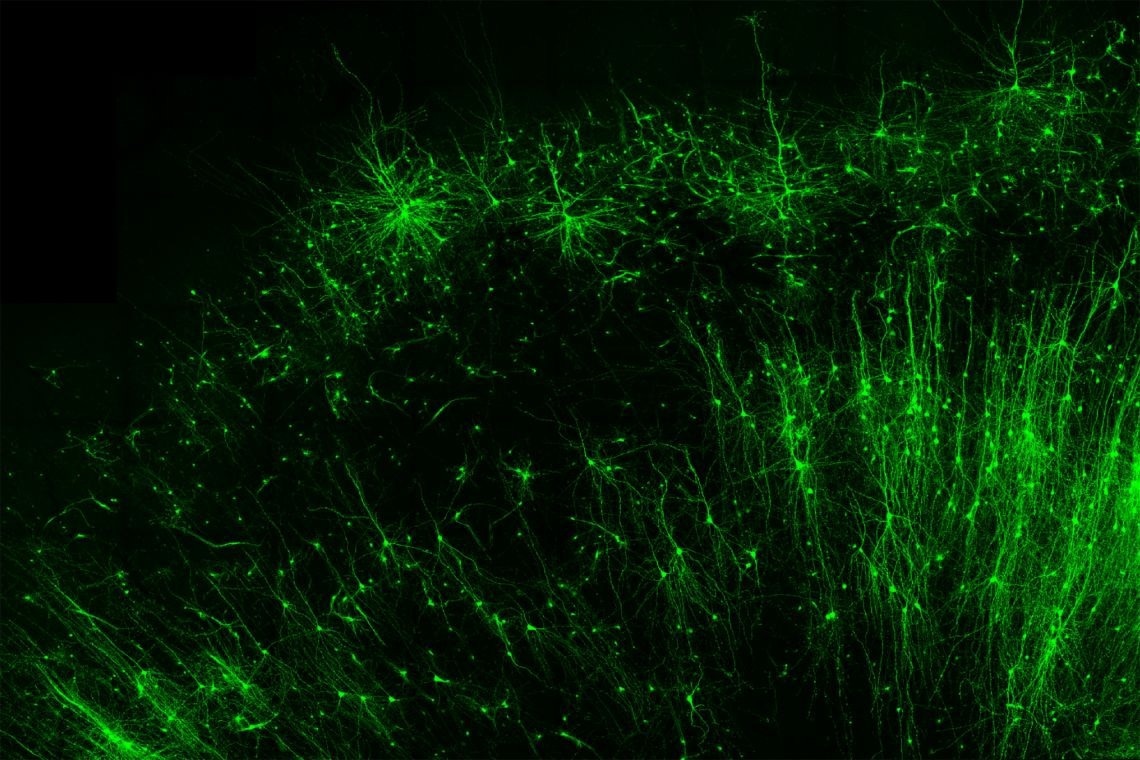 Tagging and illuminating only the inhibitory “brake” cells (green) in human brain tissue is just one of many things the new tool, CellREADR, can do. Image Credit: Derek Southwell, Duke University
Tagging and illuminating only the inhibitory “brake” cells (green) in human brain tissue is just one of many things the new tool, CellREADR, can do. Image Credit: Derek Southwell, Duke University
According to researchers, the technique could make it possible to manage disease by changing very specific cells and cell functions.
Using an RNA-based probe, a team led by Neurobiologist Z. Josh Huang, Ph.D., and Postdoctoral Researcher Yongjun Qian, Ph.D., showed they could introduce into cells fluorescent tags to label particular types of brain tissue a light-sensitive on/off switch to silence or activate neurons of their choice, and even a self-destruct enzyme to precisely expunge some cells but not others.
Nature published the article on October 5th, 2022.
Every animal’s cell contains the ADAR enzyme, which is essential to their specific cell monitoring and control system. Although CellREADR (Cell access through RNA sensing by Endogenous ADAR) is still in its infancy, Huang remarked that the prospective uses and animal kingdom compatibility appear to be infinite.
We are excited because this provides a simplified, scalable and generalizable technology to monitor and manipulate all cell types in any animal. We could actually modify specific types of cell function to manage diseases, regardless of their initial genetic predisposition. That is not possible with current therapies or medicine.”
Z. Josh Huang, Neurobiologist, Duke University
A sensor, a stop sign, and a set of blueprints are the three major components that make up the adaptable string of RNA known as CellREADR.
The research team first chooses the particular cell type that they wish to study and then locates a target RNA that is only produced by that cell type. The exceptional tissue specificity of the tool depends on the production of unique signature RNA by each type of cell.
The complementary strand of the target RNA is then constructed as a sensor sequence. RNA has the same magnetic potential to bond with another piece of RNA if it has matching molecules, similar to how the rungs on DNA are made up of complementary molecules that are naturally attracted to one another.
Both pieces of a sensor bind together to form a piece of double-stranded RNA when the sensor enters the cell and locates its target RNA sequence. This new RNA fusion causes the enzyme ADAR to examine the new product and then alter one nucleotide of its genetic coding.
All animal cells are thought to have the ADAR enzyme, a cell defense mechanism created to alter double-stranded RNA as it develops.
Realizing this, Qian used the exact same nucleotide that the ADAR edits in double-stranded RNA to create the stop sign for CellREADR. CellREADR’s sensor is very selective for a particular cell type since the stop sign that blocks the synthesis of the protein blueprints is only removed once it attaches to its target RNA sequence.
The blueprints can then be read by a cellular machinery that creates the new protein inside the target cell once ADAR removes the stop sign.
Huang and his team tested CellREADR to the limit in their study.
Huang stated, “I remember two years ago when Yongjun built the first iteration of CellREADR and tested it in a mouse brain. To my amazement, it worked spectacularly on his first try.”
The team’s meticulous planning and design paid off, as they were able to show that CellREADR successfully fitted activity monitors and control switches where needed and precisely identified individual brain cell groups in living mice. Rats and human brain tissue obtained from epilepsy procedures both responded favorably to it.
With CellREADR, we can pick and choose populations to study and really begin to investigate the full range of cell types present in the human brain.”
Derek Southwell, Study Co-Author, Neurosurgeon, and Assistant Professor, Department of Neurosurgery, Duke University
Southwell expects CellREADR will promote new therapies for neurological disorders, such as the promising new technique he is testing to treat drug-resistant epilepsy, by enhancing his and others’ understanding of the wiring schematic for human brain circuits and the cells within them.
Given that this is what first drew them both to research, Huang and Qian are especially optimistic about CellREADR’s potential as a “programmable RNA medicine” to potentially treat diseases. They have submitted a patent application for the technology.
When I majored in pharmacology as an undergraduate, I was very naïve. I thought you could do a lot of things, like cure cancer, but actually it's very difficult. However, now I think, yes maybe we can do it.”
Yongjun Qian, Postdoctoral Researcher, Duke University
Source:
Journal reference:
Qian, Y., et al. (2022). Programmable RNA sensing for cell monitoring and manipulation. Nature. doi.org/10.1038/s41586-022-05280-1