Northwestern University researchers have created a novel gene editing platform that might influence the future application of a nearly infinite library of CRISPR-based therapeutics.
CRISPR RNP (CRISPR SNA at work: in complex with single-guide RNA (red), CRISPR SNA can recognize and cleave the gene target of interest (yellow)). The complex form of Cas9 protein and single-guide RNA is referred to as the ribonucleoprotein (RNP). Image Credit: Mirkin Lab/Northwestern University
To overcome a fundamental constraint of CRISPR, the researchers used chemical design and synthesis to combine Nobel-prize-winning technology with therapeutic technologies developed in their own laboratory.
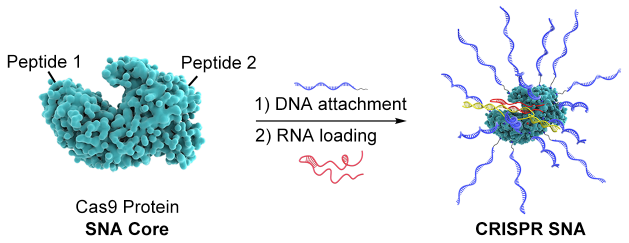
The innovative work, in particular, establishes a method for delivering the cargo essential for generating the gene-editing machine named CRISPR-Cas9. The researchers devised a method to convert the Cas-9 protein into a spherical nucleic acid (SNA) and load it with crucial components needed to access a wide range of tissue and cell types, in addition to the intracellular compartments necessary for gene editing.
The study, published in the Journal of the American Chemical Society, demonstrates how CRISPR SNAs may be transported through the cell membrane and into the nucleus while still keeping their bioactivity and capacity for gene editing.
The research, led by nanotechnology pioneer Chad A. Mirkin, builds on a 25-year effort to understand the characteristics of SNAs and what sets them apart from their well-known linear cousin, the blueprint of life.
SNAs are structures primarily made of spherical nanoparticles densely covered with DNA or RNA. Mirkin is renowned for developing these structures, which have chemical and physical characteristics that are very distinct from those of the nucleic acids found in nature.
Mirkin is the George B. Rathmann Professor of Chemistry at Northwestern University’s Weinberg College of Arts and Sciences, as well as the director of the International Institute for Nanotechnology.
Mirkin is also a professor of chemical and biological engineering, biomedical engineering, and materials science and engineering at Northwestern University’s McCormick School of Engineering, as well as a professor of medicine at the Feinberg School of Medicine.
There are numerous classes of SNAs, each with distinct chemical composition and size. SNAs are now being assessed as powerful therapeutics in six human clinical trials, including ones for deadly diseases such as glioblastoma multiforme (brain cancer) and various skin cancers.
 Image Credit: Yurchanka Siarhei/Shutterstock
Image Credit: Yurchanka Siarhei/Shutterstock
These novel nanostructures provide a path for researchers to broaden the scope of CRISPR utility by dramatically expanding the types of cells and tissues that the CRISPR machinery can be delivered to. We already know SNAs provide privileged access to the skin, the brain, the eyes, the immune system, the GI track, heart and lungs. When this type of access is coupled to one of the most important innovations in biomedical science in the last quarter-century, good things will follow.”
Chad A. Mirkin, George B. Rathmann Professor, Chemistry, Weinberg College of Arts and Sciences, Northwestern University
In this research, Mirkin’s group created a novel sort of SNA by using the protein Cas9, which is required for gene editing, as the structure’s core and attaching DNA strands to its surface. These SNAs were also coupled with peptides to modulate their ability to cross cell compartmental barriers, hence optimizing efficiency, and preloaded with RNA that could perform gene editing.
These SNAs display high gene editing effectiveness between 32% and 47% across a variety of human and mouse cell lines, just like other classes of them, and enter cells efficiently without the aid of transfection agents (which are frequently required to carry genetic material into cells).
Source:
Journal reference:
Huang, C., et al. (2022) CRISPR Spherical Nucleic Acids. Journal of the American Chemical Society. doi.org/10.1021/jacs.2c07913.