Reviewed by Danielle Ellis, B.Sc.Oct 7 2022
An important genetic discovery made by a research team led by a University of Massachusetts Amherst scientist sheds new light on the application of the drug caspofungin to treat the deadly fungal infection Aspergillus fumigatus, which claims the lives of about 100,000 severely immunocompromised individuals every year.
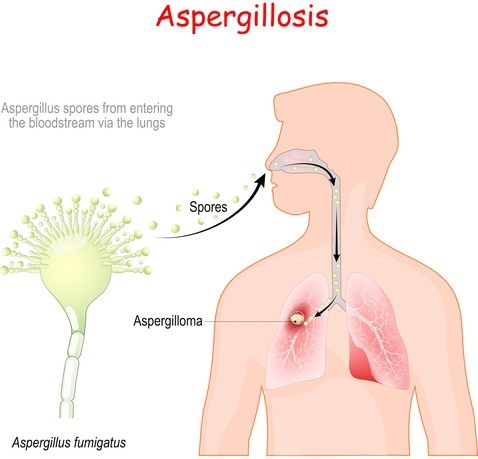 An illustration of how A. Fumigatus infects the lungs. Image Credit: Getty Images
An illustration of how A. Fumigatus infects the lungs. Image Credit: Getty Images
A. fumigatus spores are typically inhaled by healthy individuals 50 to 100 times per day when they are outside.
Our body does a great job of identifying them and destroying them.”
John Gibbons, Study Senior Author and Associate Professor, Food Science, University of Massachusetts Amherst
However, A. fumigatus can produce “a really nasty infection, invasive pulmonary aspergillosis, with a 50% mortality rate” in people whose immune systems have been damaged by cancer treatment, organ transplantation, HIV, COVID-19, and other disorders, according to Gibbons. “And there is a limited way to treat these infections.”
To further complicate matters, the anti-fungal drug occasionally has a “caspofungin paradoxical effect” [CPE], which enhances the fungal growth rather than removing it, when used as a treatment for an A. fumigatus infection.
In a study that was published in the journal Microbiology Spectrum, senior author Gibbons, former graduate student in the Gibbons lab Shu Zhao, and colleagues outline a crucial first step in the quest to comprehend when and why caspofungin treatment might be more detrimental than beneficial.
Scientists from Vanderbilt University, the University of Tennessee Science Health Center, and the University of So Paolo in Brazil made up the team that finished the first genomic and molecular identification of two genes involved in the paradoxical effect in A. fumigatus.
 Image Credit: Kateryna Kon/Shutterstock
Image Credit: Kateryna Kon/Shutterstock
The study stated, “This is one of the first studies to apply genome-wide association (GWA) analysis to identify genes involved in an Aspergillus fumigatus phenotype.”
The team used GWA, a statistical technique, to ascertain how these genetic variants are related to growth patterns at high doses of caspofungin after sequencing the genomes of 67 clinical samples, almost half of which exhibited CPE.
Gibbons added, “We identified a few candidate genes that we thought might contribute to this paradoxical effect.”
The researchers then created gene-deletion mutants using the genetic engineering technique CRISPR, which allowed them to identify two of the potential genes as being responsible for the contradictory result.
“It looks like there are many genes and many genetic variants that contribute to this phenotype. We are not done yet. One idea is that we could potentially generate new drug targets if we find the full collection of genes. We don’t understand the mechanisms yet,” further stated Gibbons.
The team’s ultimate goal is to employ DNA sequencing to study the genetic basis of many phenotypes broadly and to predict clinical advantages if an A. fumigatus patient sample carries a genotype linked to the paradoxical effect.
Gibbons concluded, “That would be an important tool that could really improve treatment.”
Source:
Journal reference:
Zhao, S., et al. (2022). Genomic and Molecular Identification of Genes Contributing to the Caspofungin Paradoxical Effect in Aspergillus fumigatus. Microbiology Spectrum. doi.org/10.1128/spectrum.00519-22