The body’s chemical processes known as “metabolism” produce the raw materials needed for development and general health.
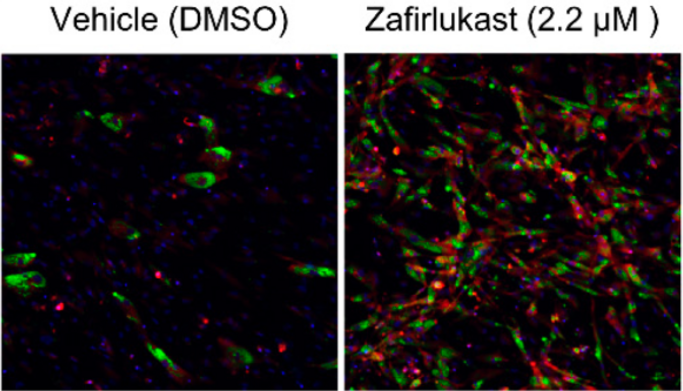 Zafirlukast induces the production of brown adipocyte tissue. For preadipocytes treated with zafirlukast (right image), brown adipocytes (shown in red) were much more prevalent than preadipocytes grown with the DMSO control (left image). Image Credit: Scripps Research and Calibr
Zafirlukast induces the production of brown adipocyte tissue. For preadipocytes treated with zafirlukast (right image), brown adipocytes (shown in red) were much more prevalent than preadipocytes grown with the DMSO control (left image). Image Credit: Scripps Research and Calibr
The substances produced and utilized during these metabolic processes are known as metabolites; nevertheless, a recent finding from Scripps Research and its drug development division, Calibr, suggests that metabolites could also be powerful molecules with the potential to treat severe diseases.
Researchers found a metabolite that changes white fat cells (often known as “bad” fat) into brown fat cells (also known as “good” fat) in a study from Metabolites that was published in August 2022 using cutting-edge drug discovery techniques.
This finding presents a prospective strategy for treating metabolic disorders such as type 2 diabetes, obesity, and cardiovascular disease. It also illustrates the possibility of discovering numerous additional potential treatments by implementing this innovative drug discovery method.
The reason many types of molecules don’t go to market is because of toxicity. With our technology, we can pull out endogenous metabolites—meaning the ones that the body makes on its own—that can have the same impact as a drug with less side effects. The potential of this approach is even evidenced by the FDA’s recent approval of Relyvrio, the combination of two endogenous metabolites for the treatment of amyotrophic lateral sclerosis (ALS).”
Gary Siuzdak, Professor, Chemistry, Molecular and Computational Biology, Scripps Research Institute
Siuzdak is also the director of the Scripps Center for Metabolomics at the Scripps Research Institute.
When the body consumes more energy than it expends, or when energy homeostasis is out of balance, metabolic diseases are frequently the result. For this reason, some therapeutic strategies have focused on transforming white fat cells (sometimes referred to as adipocytes) into brown fat cells.
Brown adipocytes convert this stored energy into heat, ultimately increasing the body’s energy expenditure and aiding in the restoration of balance. White adipocytes store excess energy and may eventually lead to metabolic diseases like obesity.
The researchers looked through Calibr’s ReFRAME drug-repurposing collection—a library of 14,000 recognized drug compounds that have been FDA-approved for various diseases or have undergone thorough human safety testing—to find a treatment that could promote the formation of brown adipocytes.
The researchers screened ReFRAME for a drug with these particular properties using high-throughput screening, an automated drug discovery method for looking through enormous pools of information.
They discovered zafirlukast, an asthma drug that has FDA approval, in this way. They established that zafirlukast could convert white adipocytes into brown adipocytes and adipocyte precursor cells, or preadipocytes, into primarily brown adipocytes through a series of cell culture tests.
Although a positive discovery, zafirlukast is hazardous when used in excessive dosages, and it was not completely obvious how the drug was altering fat cells. At that point, the researchers teamed up with Siuzdak and his group of metabolite specialists.
We needed to use additional tools to break down the chemicals in zafirlukast’s mechanism. Framed another way, could we find a metabolite that was providing the same functional effect that zafirlukast was, but without the side effects?”
Kristen Johnson, Study Co-Senior Author and Director, Translational Drug Discovery Research, Calibr
To address Johnson’s question, Siuzdak and his group developed a new series of tests known as drug-initiated activity metabolomics (DIAM) screening.
To sort through hundreds of molecules and pinpoint certain metabolites, DIAM makes use of technologies like liquid chromatography, a tool that separates components in a mixture, and mass spectrometry, an analytical method that separates particles by weight and charge.
In this instance, the researchers were looking for metabolites in adipose tissue that would promote the development of brown adipocyte cells.
They discovered myristoylglycine—an endogenous metabolite that stimulated the formation of brown adipocytes without causing harm to the cell—after narrowing down 30,000 metabolic characteristics to only 17 metabolites.
Even among almost structurally identical metabolites, only myristoylglycine possessed this unique property out of the thousands of metabolic parameters examined during the investigation.
Johnson added, “Identifying myristoylglycine among the thousands of other molecules speaks to the power of Siuzdak’s approach and these technologies. Our findings illustrate what happens when an analytical chemistry team and a drug discovery group closely collaborate with each other.”
Source:
Journal reference:
Guijas, C., et al. (2022). Drug-Initiated Activity Metabolomics Identifies Myristoylglycine as a Potent Endogenous Metabolite for Human Brown Fat Differentiation. Metabolites. doi.org/10.3390/metabo12080749