If text-based artificial intelligence image generator like Craiyon or DALL-E has ever been used, it would lead to the knowledge of lifelike, yet entirely, synthetic visuals that AI tools produce.
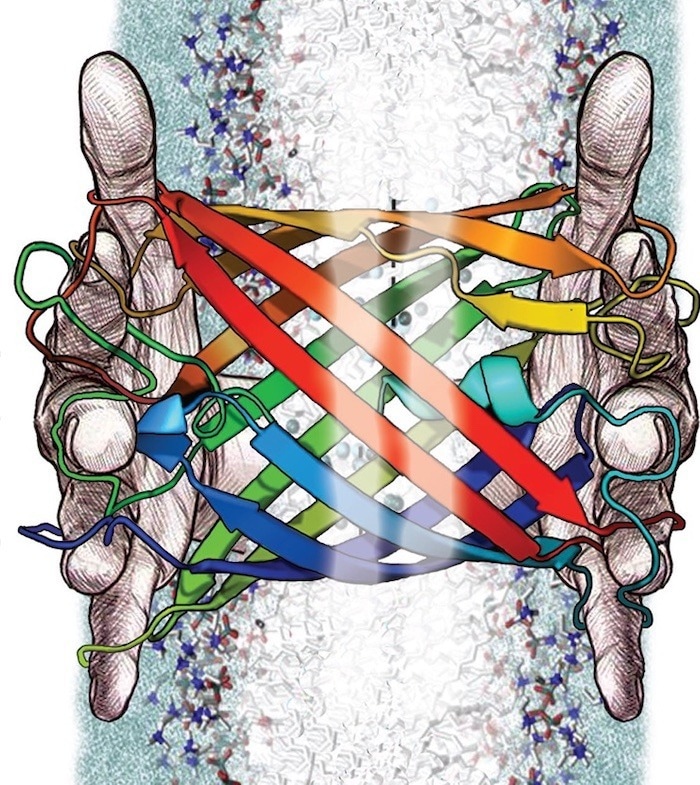 A University of Kansas researcher will use machine learning to create “deep-fake” membrane beta-barrel proteins—a class of naturally successful biosensors—designed to detect polluting metal ions in water. Image Credit: T. Chris Gamblin
A University of Kansas researcher will use machine learning to create “deep-fake” membrane beta-barrel proteins—a class of naturally successful biosensors—designed to detect polluting metal ions in water. Image Credit: T. Chris Gamblin
Such websites use machine learning to scan millions of online images, evaluate them, and piece together parts of them to create new but false ones.
Now, scientists at the University of Kansas are aiming to create new proteins intended to identify water pollutants using a similar machine-learning method.
The Molecular Foundations for Biotechnology program of the National Science Foundation has awarded a new three-year, $1.5 million grant to a KU researcher who will use machine learning to develop “deep-fake” membrane beta-barrel proteins, a class of naturally effective biosensors that are intended to detect polluting metal ions in water.
These beta barrels are super useful because they can bring things across membranes. Barrels make good enzymes—there are so many different things that barrels can do.”
Joanna Slusky, Study Principal Investigator and Associate Professor, Molecular Biosciences, The University of Kansas
The binding characteristics of the tube-shaped beta barrels have been changed for a range of applications by prior research. However, a large portion of this laborious effort had to be done by hand, frequently leading to slight variances in a small set of scaffolds or barrel constructions.
Slusky added, “In this case, we are using machine learning to generate large numbers of barrels. But, how about if we can both generate barrels and have them be useful? We asked ourselves, ‘What’s a biotechnology application of barrels?’ Well, one would be metal sensors that could perhaps detect metal pollutants.”
Slusky will create a new machine-learning process that generates beta-barrels with scaffolds identical to those found in nature, but with different sequences, in collaboration with her co-principal investigators, professors Rachel Kolodny and Margarita Osadchy of Haifa University in Israel, and postdoctoral fellow Daniel Montezano of the University of Kansas.
“There is a website called ‘This X Does Not Exist.’ If you go to that site, you see all these AI-generated things and people don't really exist. But a computer made an image, for instance, of a cat. But that's not really a cat—a computer took a bunch of pictures of cats and said OK, we can just sort of generate as many cat pictures as you want now, because we figured out what is a cat.’ We need to make something real so we see it more like generating a recipe,” added Slusky.
She further stated, “The question is, how to make computers generate a recipe for proteins.”
Since “natural proteins are sort of a small blip in the number of possible sequences,” beta barrels are well-suited to progress through machine learning.
According to Slusky, if a computer algorithm can figure out the essentials of what makes a protein what it is, it will stop producing pointless sequences.
She pointed out, “Most sequences would never actually be proteins—they wouldn’t have a particular fold. They would just kind of bond with themselves in weird, nonpredictable ways over and over again. To be a protein, you need a sequence that makes one shape. When people tried to make random sequences, or even somewhat directed sequences, they found that only a very, very small percentage of them might actually be a protein.”
Slusky and her coworkers plan to produce a beta-barrel that is particularly well-suited to identifying metal ions in water using machine learning to create novel and viable sequences that lead to this common fold. The effort will lead to the development of biosensors based on beta barrels that can detect toxins like lead in waterways.
Slusky added, “If we make them the right size, this molecule will be ideal to put some particular metal in, and you can have the right substituents so that it would bind that metal. Because it is in a membrane, it can give you some sort of conductance difference—there is a difference between when it's bound and when it is not bound.”
“If you are able to do that, you could sense for different metals, and different concentrations of those metals. There are a lot of big steps we want to accomplish, but I am hopeful and excited,” stated Slusky.
Slusky’s teaching at KU and the outreach to high-school science students will both benefit from the work, in addition to the training of undergraduate researchers in her lab.