Reviewed by Danielle Ellis, B.Sc.Oct 26 2022
Not just three, but even five proteins share important roles in the formation and function of synapses and can substitute for each other. This discovery was made by a team of the research focus "Mental Health & Neuroscience" of the Karl Landsteiner University of Health Sciences Krems (KL Krems) and the CavX PhD program of the Medical University of Innsbruck. Most of these proteins are components of so-called calcium channels, and only recently the team had succeeded in discovering redundant functions for three of the proteins in synapse formation and neuronal signal transmission. The recent finding, that two additional proteins (α2δ-4 and Cachd1) can fulfill the same functions is surprising and raises questions about the evolution of the nervous system.
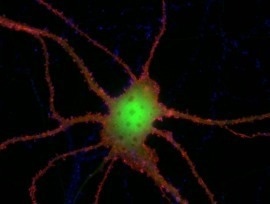
Image Credit: KL/C. Ablinger und G. Obermair
Ion channels serve to conduct signals in the nervous system, so it is essential that their function is tightly regulated. Proteins of the α2δ-family (pronounced "alpha-two-delta") play an important role in this process. They serve as regulatory subunits of calcium channels and hence for a long time they are known to regulate calcium currents. Recently, however, Univ. Prof. Dr. Gerald Obermair, head of the Division of Physiology at the KL Krems, and his team were able to show that three of the four α2δ-proteins are also chiefly involved in the formation of synapses and that they can substitute for each other in this fundamental function. This caused a considerable stir, as α2δ-proteins are associated with diseases such as epilepsy, autism, schizophrenia, and anxiety. Now Prof. Obermair and his research group have succeeded in showing that the last of the four proteins of this family and also another protein not only influence synapse formation, but also affect signal transmission.
Two is better. many are best
In contrast to the previously studied α2δ-proteins (isoforms -1, -2, -3), the now investigated α2δ-4 occurs predominantly in the retina of the eye but is hardly found in the brain. The current results, as Prof. Obermair explains, are even more surprising: "We were able to show in cell cultures that α2δ-4 can exert very similar functions in the brain as the previously studied proteins α2δ-1 to -3. Indeed, all these proteins can even replace each other in their most critical function. This seems wasteful and is remarkable in evolutionary terms."
On top of that, the team also studied a protein known as Cachd1. While this protein is structurally similar to the α2δ-proteins, it is still unclear whether it also serves as a subunit of ion channels. Unlike α2δ-4, however, it is abundantly found in the brain and has been linked to brain functions. This and its similarity to α2δ-proteins were reasons enough to take a closer look at the functions of Cachd1.
And indeed. It turned out that Cachd1 can also take over the functions of α2δ-proteins. Hence, it can modulate synapse formation and also affect channel function.
Cornelia Ablinger, Study First Author and Student, CavX PhD Program
Further experiments with all α2δ isoforms and Cachd1 showed that the ability to substitute for each other does not come without subtle differences. For example, analyses of synaptic calcium signals identified minute differences indicating specific modulatory roles of each protein. A finding that allows Prof. Obermair to speculate on the apparent redundancy of the proteins: "It may well be that in the course of evolution they diversified one after the other to adapt the critical control of nerve signal transmission to the requirements of increasingly complex organisms."
Experimental challenge
The surprising results were only made possible by ten years of preliminary work by Prof. Obermair’s team. Actually, it was the ability of α2δ proteins to substitute for each other that posed an experimental challenge. Particularly, because first a cellular nerve cell model had to be developed in which all three genes for α2δ-1 to -3 were switched off. This endeavor turned out to be a big challenge, with a success rate of less than 5 percent.
However, once the team – which received a great deal of international attention – had overcome this hurdle, the questions could be experimentally tackled. The discovery that also α2δ-4 and Cachd1 can modulate synapse formation and differentiation was made possible by the preliminary work. Precise measurements of calcium signals of individual synapses provided evidence that α2δ-4 and Cachd1 can also modulate channel function. Following up on these questions, further cutting-edge research results can be expected at the research focus "Mental Health & Neuroscience" at the KL Krems.
Source:
Journal reference:
Ablinger, C., et al. (2022) α2δ-4 and Cachd1 Proteins Are Regulators of Presynaptic Functions. International Journal of Molecular Sciences. doi.org/10.3390/ijms23179885.