According to a recent study, which will be published on November 1st, 2022, by Yun-Fei Li of Zhejiang University School of Medicine in Hangzhou, China, and colleagues, a decrease in protein synthesis in growing gut cells contributes to a rare genetic condition, and an affordable nutritional supplement could help reverse that decrease.
 Phenotypic analysis of Mycn mutants. Morphology of the whole intestine visualized via HE staining for the WT and mycn mutant embryo sections at 4 dpf. Black arrows indicate goblet cells in the WT intestines. Sections were cut along the sagittal plane. Image Credit: Li Y-F et al., 2022, PLOS Biology, CC-BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
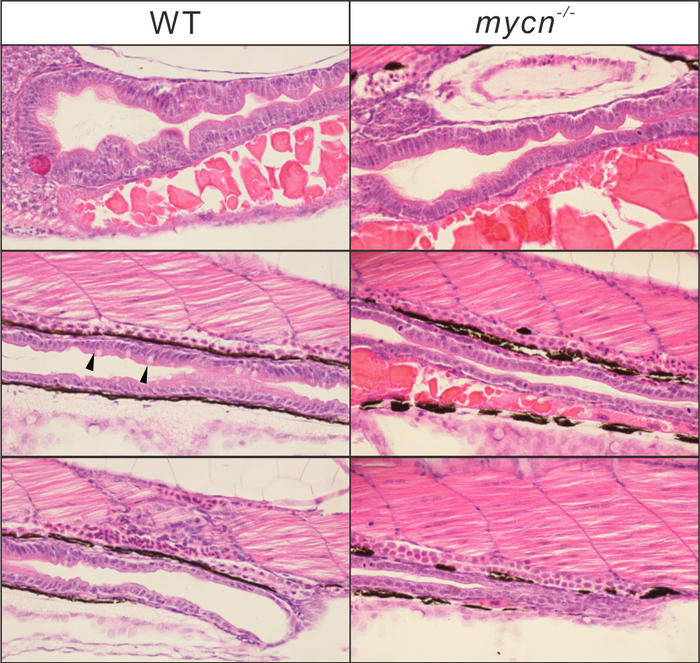
Phenotypic analysis of Mycn mutants. Morphology of the whole intestine visualized via HE staining for the WT and mycn mutant embryo sections at 4 dpf. Black arrows indicate goblet cells in the WT intestines. Sections were cut along the sagittal plane. Image Credit: Li Y-F et al., 2022, PLOS Biology, CC-BY 4.0 (https://creativecommons.org/licenses/by/4.0/)
The discovery advances the comprehension of the disease’s pathophysiology and could result in new treatments.
Feingold syndrome type 1 causes a variety of developmental issues, including issues with the skeleton and neurological system, but intestinal atresia, or inadequate development of the gastrointestinal tract, has the biggest influence on patients’ life.
There is currently no animal model to examine the implications of the loss-of-function mutations that cause the condition in the Mycn gene, which encodes a crucial transcription factor that controls the activity of many genes.
By utilizing CRISPR genome editing to remove a section of the Mycn gene in zebrafish, whose gut development has striking similarities to that of humans, the investigators were able to generate that model. They discovered that the loss of gene activity, as a result, resulted in a drastic reduction in the size of the intestine, both in terms of length and in terms of the folding that provides the intestine with its enormous surface area for absorption.
They discovered a considerable down-regulation of many ribosomal genes within a particularly afflicted subgroup of cells in the developing gut, which lowered gene translation and protein synthesis.
Genes involved in the mTOR signaling pathway, a crucial regulator of protein synthesis, were particularly impacted; treating wild-type zebrafish with an mTOR inhibitor reproduced the intestinal developmental flaws observed in Mycn mutants.
The intestinal size of the mutant fish partially returned to normal after the authors gave them leucine, an amino acid known to stimulate the mTOR pathway.
The study corresponding authors, Peng-Fei Xu and Xi Jin, stated, “Our work shows that during embryonic development, intestinal cells, which are in a highly proliferative state, require high Mycn expression levels. And that the proliferation arrest caused by reduced protein synthesis was the main reason for the developmental defects in the intestines of the Mycn mutant.”
They also added, “This suggests a possible treatment strategy for the intestinal symptoms in patients with Feingold syndrome type 1, although confirmation in a human intestinal organoid system is essential.”
The study first authors, Yun-Fei Li and Tao Cheng, further stated, “Feingold syndrome resulting from Mycn deficiency has been discovered for decades, however, the mechanism that leads to gastrointestinal atresia in Feingold syndrome type 1 is still unclear. We developed a Mycn mutant zebrafish model which recapitulates key phenotypes of Feingold syndrome type 1, and also provided a possible treatment strategy for Feingold syndrome type 1.”
Source:
Journal reference:
Li, Y.-F., et al. (2022). Mycn regulates intestinal development through ribosomal biogenesis in a zebrafish model of Feingold syndrome 1. PLoS Biology. doi.org/10.1371/journal.pbio.3001856