In a recent study, scientists from the University of Maryland have explained how actin, a protein, plays a key role in giving cells their shape.
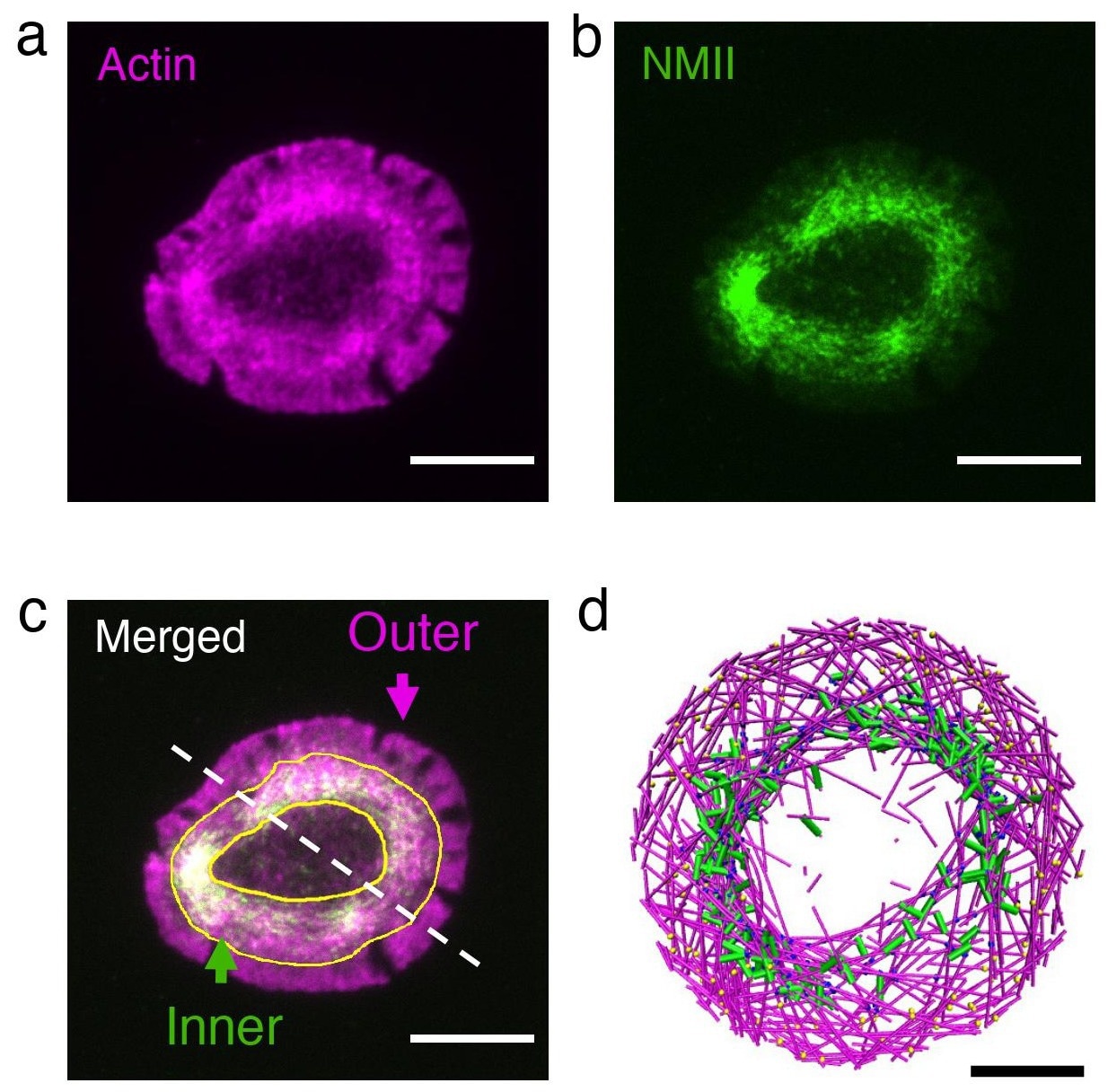 Actin (shown in magenta and in box “a”) and myosin (shown in green and in box “b”) are depicted in the actin rings of live T cells (box “c”). Box “d” provides a snapshot of MEDYAN simulations, which resemble the actin ring found in T cells. Image Credit: Haoran Ni
Actin (shown in magenta and in box “a”) and myosin (shown in green and in box “b”) are depicted in the actin rings of live T cells (box “c”). Box “d” provides a snapshot of MEDYAN simulations, which resemble the actin ring found in T cells. Image Credit: Haoran Ni
In a manner similar to how our skeletons support our bodies, actin is an essential part of the cytoskeleton that gives cells structure. The actin cytoskeleton, in contrast to our skeleton, is a very elastic structure that can quickly come together and fall apart in response to biochemical and biophysical signals.
Actin can both form 2D rings that alter intracellular processes and 3D spherical shell-like structures that shield cells from pressure. However, if scientists attempted to duplicate similar structures outside of the cell, they almost always came up with actin clusters. Until now, no one was aware of the cause.
The researchers demonstrated through computer simulations that actin and myosin, the protein that it partners with, engage in a tug-of-war, with actin attempting to escape and myosin attempting to confine it in local clusters.
If actin triumphs, actin filaments are freed from myosin’s grip and form spherical shells and rings on their own. Actin network collapses and creates dense clusters if myosin prevails.
Actin rings and spherical shells are ubiquitous in almost all cell types across species. We think that understanding the mechanism behind the formation of these structures unlocks the door to how cells sense and respond to their environment.”
Garegin Papoian, Study Co-Author and Monroe Martin Professor, Department of Chemistry and Biochemistry, University of Maryland
Their findings, which were released on October 21st, 2022 in the journal eLife, could have significant effects on human health. The results of this study could help the creation of future medications because actin rings are crucial to our bodies’ capacity to fight off foreign cells, with abnormalities potentially leading to decreased immunity or autoimmune diseases.
Actin filaments, which resemble trains, are created when actin monomers join together. The treadmilling mechanism causes these actin trains to move throughout the cell. The myosin motors, which direct trains in opposing directions toward one another, are also in action.
According to Papoian, Qin Ni (PhD ‘21, chemical engineering), and PhD student in biophysics Haoran Ni, the creation of actin rings was caused by a conflict between the pulling force of myosin and the rate of treadmilling.
Since it is impossible to fine-tune these parameters in living cells, the researchers used a simulation program called MEDYAN, created by the Papoian Lab. MEDYAN simulates the movements of cytoskeletal proteins by applying physics and chemical laws. They created a thin disc and spherical shell to replicate an actin and myosin network (also known as actomyosin).
They discovered that actin trains that move slowly experience traffic jams, or actomyosin clusters, that have been seen in networks assembled outside of cells. However, if the actin trains travel quickly, they can elude the pull of myosin.
When the actin trains get close to the disc’s edge, myosin’s pulling force causes them to pivot, preventing a collision with the edge. All the trains move in a circle around the disc’s edge as a result of the repetition of these occurrences, forming the actin ring.
A thermodynamic hypothesis is presented in further study to explain why cells form rings and shells. The lowest energy configuration is preferred by systems, according to the rules of physics. Actin filaments are bent by myosin proteins, which store mechanical energy that must be released before actin can unwind.
In living cells, the development of rings or shells, which are the lowest energy structure according to thermodynamics, is made possible by actin's ability to move quickly enough to avoid myosin and run to the edge.
Papoian added, “The reason rings were not previously seen outside the cell is because actin just was not moving fast enough. Myosin was winning 10 times out of 10.”
The researchers focused on T cells, where rings naturally form, to verify this model in living cells with the assistance of Professor Arpita Upadhyaya, physics graduate student Kaustubh Wagh, biological sciences graduate student Aashli Pathni, and biophysics graduate student Vishavdeep Vashisht.
Human bodies’ T cells are the ones that look for foreign cells. The T cell cytoskeleton quickly rearranges itself to produce an actin ring at the cell-cell interface when activated and upon recognizing a cell as foreign. Using high-resolution live-cell imaging, the researchers first examined the impact of perturbing actin and myosin in cells that had formed rings.
In remarkable agreement with related models, slowing down the actin train caused the ring to break up into small clusters, while speeding up myosin contraction caused the ring to rapidly constrict.
The team intends to expand the model’s complexity and incorporate additional cytoskeletal elements and organelles as a follow-up to this study.
“We have been able to capture one fundamental aspect of cytoskeletal organization. Piece by piece, we plan to build a computational model of a complete cell using fundamental principles from physics and chemistry,” stated Papoian.
Source:
Journal reference:
Ni, Q., et al. (2022). A tug of war between filament treadmilling and myosin induced contractility generates actin ring. eLife. doi.org/10.7554/eLife.82658