To create new cells, the old cells must divide. This is a constant, frequent, and pervasive process that begins with conception and culminates with death. There are an estimated 37 trillion cells in the human body’s tissues and organs, each of which is the result of one cell dividing into two.
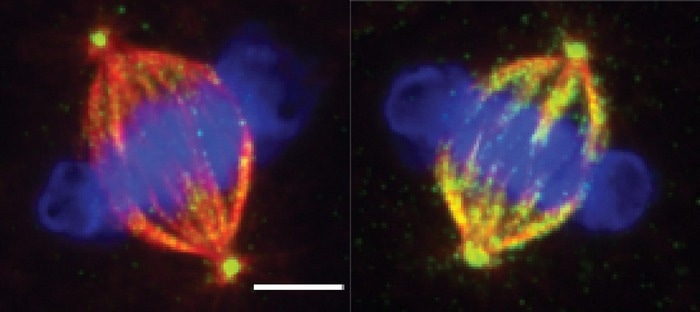 Mitotic spindles with microtubules (red) attached to chromosomes (blue) during cell division. Cells with normal TTLL11 function (right) have normal rates of microtubule polyglutamylation (green) while cells without TTLL11 (left) are unmodified. Image Credit: Centre for Genomic Regulation.
Mitotic spindles with microtubules (red) attached to chromosomes (blue) during cell division. Cells with normal TTLL11 function (right) have normal rates of microtubule polyglutamylation (green) while cells without TTLL11 (left) are unmodified. Image Credit: Centre for Genomic Regulation.
Aneuploidy occurs when cell division goes wrong, resulting in the formation of new cells with an incorrect number of chromosomes. Chromosomal instability (CIN) refers to the frequency with which chromosome segregation mistakes occur.
In some situations, such as with a growing embryo, this can encourage spontaneous abortion, while in others, it can contribute to human diseases like cancer. Aneuploidy and CIN are characteristics of aggressive tumors.
Cell division is studied by researchers to learn how chromosome segregation occurs without errors every time a cell divides. They also look at why and how mistakes happen and how aneuploid cells form.
The precise attachment of the chromosomes to the mitotic spindle, the molecular machinery that draws chromosomes to opposite ends of a cell, is completely dependent on faithful chromosome segregation.
The spindle is made up of microtubules, which are long, hollow tubes made of proteins. Microtubules must be sturdy enough to move and align chromosomes while remaining dynamic enough to allow for the repair of incorrect attachments before chromosomes are torn apart and segregated.
Understanding how cells divide relies on understanding the dynamics of microtubules and how they are finely regulated. Their significance is highlighted by the discovery in the early 1970s of a compound that directly stabilizes microtubules, allowing cancer cells to self-destruct, and is still used in chemotherapy today.
According to ICREA Research Professor Isabelle Vernos, Group Leader in the Quantitative Cell Biology research program at the Centre for Genomic Regulation (CRG), the regulation of microtubule dynamics during cell division in cancer is currently unknown and could offer novel pathways to exploit. Her most recent work, published in Nature Communications, identifies potential new effective cancer treatment targets.
In various types of cells and tissues, microtubules undergo a variety of changes that finely define their specialized roles. The researchers discovered tubulin tyrosine ligase like 11, commonly known as TTLL11, an enzyme that particularly alters spindle microtubules by adding glutamate chains to the microtubule surface.
They discovered that microtubule polyglutamylation specifies the dynamism and stability of spindle microtubules, which in turn secures the faithful segregation of the chromosomes. Time-lapse imaging demonstrated that cells and zebrafish embryos were substantially more likely to have chromosomal segregation abnormalities in the absence of TTLL11.
When the researchers compared TTLL11 levels in cancer to healthy tissue utilizing data from the Cancer Genome Atlas, a public database that comprises molecular characteristics of over 20,000 primary cancer samples matched with normal samples covering 33 cancer types, they discovered that TTLL11 was considerably downregulated in every single one of these tumors compared to the corresponding normal tissue.
We found that low levels of TTLL11 are highly specific to cancer. This is exciting because it reveals another layer to how microtubules are regulated during cell division in cancer. Drugs that interfere with microtubule dynamics are already one of the most successful first-line cancer therapies. If this turns out to be a viable therapeutic target, our findings pave the way to the creation of a new generation of drugs that are more precise and effective than conventional treatments.”
Isabelle Vernos, Group Leader, Quantitative Cell Biology Research Program, Centre for Genomic Regulation
The study’s authors also revealed why low TTLL11 levels impact cell division.
Microtubules need to establish stable connections with the chromosomes to align them and pull them apart, but these connections also need to be flexible enough to be corrected on time to avoid any errors during segregation. Microtubules in cancer cells have low levels of TTLL11, making them too stable thereby favoring the segregation of chromosomes in the presence of attachment errors that will result in aneuploid cells.”
Isabelle Vernos, Group Leader, Quantitative Cell Biology Research Program, Centre for Genomic Regulation
“It remains to be demonstrated whether this is a mechanism being exploited by cancer cells to grow and generate diversity through random chromosome segregation errors. This constitutes new exciting avenues of research,” concludes Dr Vernos.
Source:
Journal reference:
Zadra, I., et al. (2022) Chromosome segregation fidelity requires microtubule polyglutamylation by the cancer downregulated enzyme TTLL11. Nature Communications. doi.org/10.1038/s41467-022-34909-y.