Polymorphs are solid materials that can exist in different crystallographic structures with varying crystal lattice arrangements.1
Polymorphism is the underlying chemistry that allows the substance to be in more than one crystalline form. Polymorphs have wide-ranging applications, including pharmaceuticals, pigments, dyes, and foods.
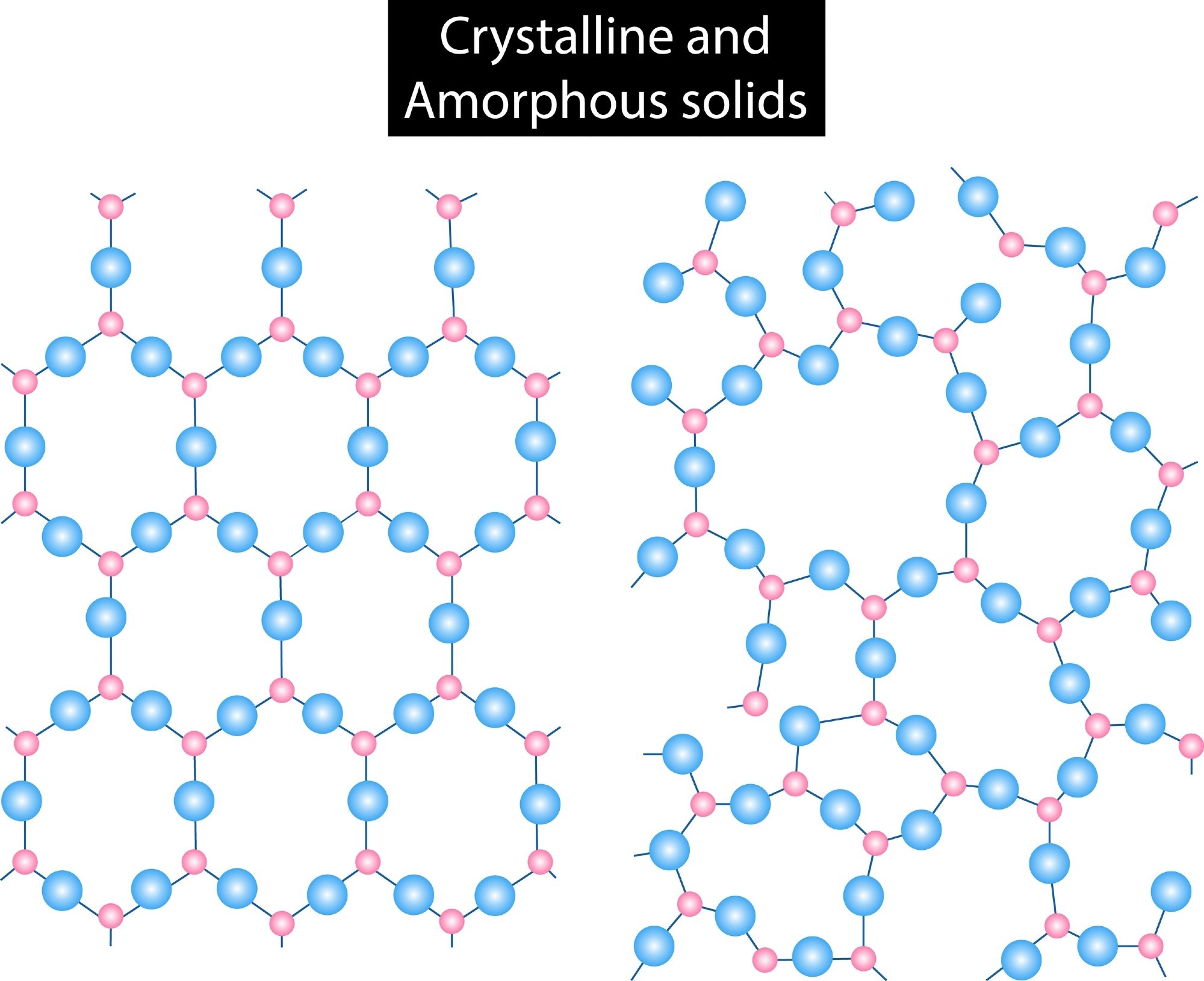
Image Credit: Jo Sam Re/Shutterstock.com
Polymorphs: Definition and Types
In 1899, Ostwarld stated, “Almost every substance can exist in two or more solid phases provided the experimental conditions are suitable.”2 This implies that the physiochemical properties of a compound can be changed based on different polymorphs. In 1965 W.C. McCrone defined polymorph as “A solid crystalline phase of a given compound resulting from the possibility of at least two different arrangements of the molecules of that compound in the solid state”.3
Polymorphism causes differences in physical properties with different molecular arrangements. During drug development, altering the physical forms of polymorphs can achieve favorable physicochemical properties of compounds, such as bioavailability, solubility, dissolution, and physical/chemical stability.
Polymorphs have been divided into two subtypes, namely, conformational polymorphs and packing polymorphs. Conformational polymorphs are formed when conformationally different molecules exist in the crystalline state while packing polymorphs are formed when molecules share the same molecular conformations with different arrangements in three-dimensional (3D) space.2
A polymorph could be either monotropic or enantiotropic. Monotropic polymorphs are stable over an entire temperature range. In contrast, in enantiotropic polymorphs, one form is stable below the transition temperature, and the other is stable above the transition temperature.
Key Theories of Polymorph Formation
In nature, every compound tends to shift to achieve a thermodynamically stable state, for which it might undergo molecular rearrangement to attain the most stable state. Scientists have formulated several theories of polymorph formation. According to Ostwald’s theory, when a system leaves any state, it tends to transit to a more stable state, not the most stable one but the nearest one under a given condition.2
Polymorphs also form via cross-nucleation. This phenomenon mostly occurs in small organic molecules and polymers from a solution. Several different polymorphs nucleate on each other during the cross-nucleation process.
Typically, this process is affected by thermostability, lattice matching, and crystallographic orientation of the initial polymorph formed. Carefully designed additives can inhibit nucleation. Growth inhibition in specific faces could influence the morphology and dissolution rate of a polymorph.4
What are Nucleoside Analogs?
Polymorph Preparation Methods and Analysis
Multiple studies have shown that polymorphs are created by changing external conditions, such as temperature, pressure, and even particle size.5 During drug discovery, it is important to select the suitable polymorph to attain the desired stability and physicochemical properties.
Different methods are used to develop polymorphs, including crystallization from a single or mixed solvent, thermal activation of the solid substrates, crystallization from the melt, dehydration of solvates, seeding, and slurry conversion method.6
Polymorphs are formed by evaporation or antisolvent crystallization of single or mixed solvents. Selection of suitable solvents for the target polymorph formation could be challenging. Typically, the solvents are selected based on their physicochemical properties.
Solvents are classified into different categories based on their hydrogen-bond acceptor/donor propensity, dipole moment, polarity/dipolarity, dielectric constant, and other variables.
Polymorph formation also depends on the crystallization temperature, evaporation rate, degree of supersaturation, pH of the media, and the rate of agitation. In the thermal activation method, it is important to understand the thermodynamic relationships between polymorphs to develop a desired polymorph.2
Polymorphs are also formed via the desolvation or dehydration method. Solvates undergo a phase transition and form non-solvated/anhydrous polymorphs. It may also lose crystallinity and form amorphous material.
Based on the external stress applied to amorphous materials, crystallization from the melt produces multiple polymorphic forms with different kinetics and mechanisms.
Solution mediate polymorphic transformation (SMPT) is an easy, rapid, and reliable method to form stable polymorphs. SMPT occurs through the dissolution of the metastable form followed by nucleation or growth of another form, which is more stable from the initially formed polymorphs.
Polymorphs are analyzed using solid-state characterization techniques, including powder X-ray diffraction (PXRD) equipped with Cu Kα radiation, differential scanning calorimetry (DSC), X-ray diffraction (XRD), microscopic technique, and infrared spectroscopy.6
Image Credit: Monika Gruszewicz/Shutterstock.com
Polymorphs Applications
Polymorphism plays an important role in drug discovery and development. It is important to assess the structure of the solid crystal to determine the drug's effectiveness. Typically, drugs with low water solubility exhibit variable bioavailability and poor clinical response, and these can be resolved by achieving stable polymorph formation.7
Paracetamol (PCA), also known as acetaminophen, is a commonly used analgesic that exhibits three polymorphic forms, I, II, and III. Commercially used acetaminophen polymorph is form I. Acetaminophen form II was found to be more soluble than form I, but is less stable. This form is developed by crystallizing solids in benzyl alcohol at high temperatures or by evaporation method.8
Ritonavir is a protease inhibitor used to treat HIV-1 infection. This drug was first marketed in 1996 and soon withdrawn due to the sudden appearance of the less soluble form II Norvir semi-solid capsules. Based on multiple experiments, form II was found to be the most stable among the five polymorphic forms isolated so far.9
In the food industry, the polymorphic state of fat is considered to be a quality indicator of the lipid phase in the food product. For example, the cocoa butter in chocolate should be crystallized in the β2 polymorphic form to obtain the desired gloss and melting range. Mostly, the X-ray diffraction (XRD) method is used to determine the polymorphic state and assess the chocolate quality.10
References
- Nogueira BA, et al. Color polymorphism in organic crystals. Commun Chem. 2020;3, 34. doi.org/10.1038/s42004-020-0279-0
- Lee EH. A practical guide to pharmaceutical polymorph screening & selection. Asian J Pharm Sci. 2014; 9(4), 163-175. doi.org/10.1016/j.ajps.2014.05.002
- Lombardo GM, Punzo F. (False asymmetry, pseudosymmetry, disorder, polymorphism and atomic displacement parameters. J Mol Struct. 2014; 1078, 158-164. doi.org/10.1016/j.molstruc.2014.03.057
- Evans JS. Polymorphs, Proteins, and Nucleation Theory: A Critical Analysis. Minerals. 2017; 7(4):62. doi.org/10.3390/min7040062
- Cruz-Cabeza AJ, et al. Open questions in organic crystal polymorphism. Commun Chem. 2020; 3, 142. doi.org/10.1038/s42004-020-00388-9
- Liang C. Organic Polymorphs Based on AIE-Active Molecules: Preparation,Characterization, and Application. Cryst. Growth Des. 2024; 24, 7322−7341.doi.org/10.1021/acs.cgd.4c00499
- Censi R, Di Martino P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules. 2015;20(10):18759-76. doi: 10.3390/molecules201018759..
- Yeh KL, et al. Crystallization of Form II Paracetamol with the Assistance of Carboxylic Acids toward Batch and Continuous Processes. Pharmaceutics. 2022;14(5):1099. doi: 10.3390/pharmaceutics14051099.
- Bauer J, et al. Ritonavir: an extraordinary example of conformational polymorphism. Pharm Res. 2001;18(6):859-66. doi: 10.1023/a:1011052932607.
- Declerck A, et al. Characterisation of Fat Crystal Polymorphism in Cocoa Butter by Time-Domain NMR and DSC Deconvolution. Foods. 2021;10(3):520. doi: 10.3390/foods10030520.
Further Reading
Last Updated: Feb 27, 2025